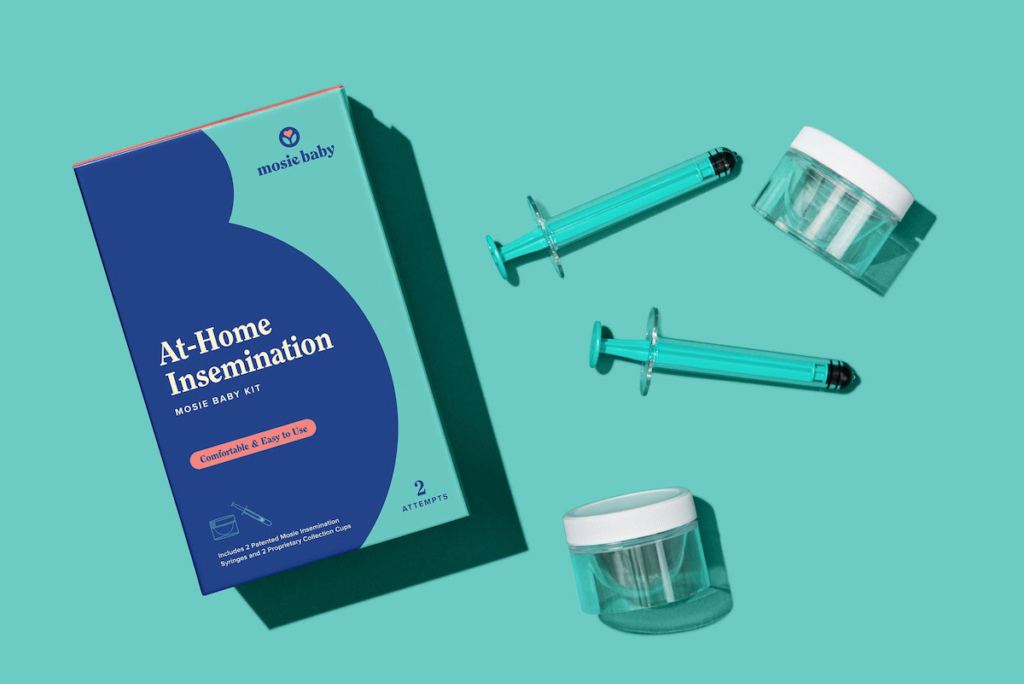
Mosie Baby, an at-home fertility care company, has recently obtained FDA Class II clearance for its Mosie Baby Kit. This clearance positions the Mosie Baby Kit as the first and only over-the-counter option for intravaginal insemination (IVI) approved by the FDA.
The kit was developed to help individuals and couples who face challenges with natural conception or for whom traditional intercourse is not an option. With the recent FDA 510k Class II clearance, the kit, which has been shown to be “substantially equivalent” to devices used in clinically-administered intrauterine insemination (IUI), is poised for broader accessibility.
Each kit contains two patented syringes designed specifically for at-home insemination and two proprietary collection cups for semen collection. The syringe features a unique barrel-free tip and slit opening designed to optimize transfer and minimize waste, while the collection cup is tailored for maximal semen collection. The FDA clearance process involved thorough clinical and technical testing, including Human Sperm Survival Assay, vaginal irritation testing, and biocompatibility testing, ensuring that the device is safe, non-toxic, and free from microbial contamination.
“Nearly 10 years ago, my husband and I were devastated by a diagnosis of unexplained infertility and were desperate for options that were safe, financially accessible and easy to use at home,” said Co-founder and CEO Maureen Brown. “Since inventing the Mosie Baby Kit in 2014, we realized we weren’t alone in our fertility journey as it’s reported that one in six people experience infertility. To date, we’re very proud to share that Mosie Baby has helped more than 100,000 families inseminate from the comfort of their own home. We are now thrilled to offer our device as an FDA reviewed option for families looking to inseminate at home.”
“The recent Mosie Baby clearance means hundreds of thousands of underserved people now benefit from an at home option. The technology adheres to the highest FDA recognized test standards and is supported by robust clinical performance testing. The team took the time and care to manufacture a quality product. I am delighted to see it become accessible to countless future parents on their fertility journey. The team at Mosie Baby is on a path to becoming the at home gold standard fertility option.” Kwame Ulmer, Managing Partner at MedTech Impact Partners and former Deputy Director at FDA.
Earlier this year Mosie Baby announced a $1M raise to support the company’s retail expansion plans.



