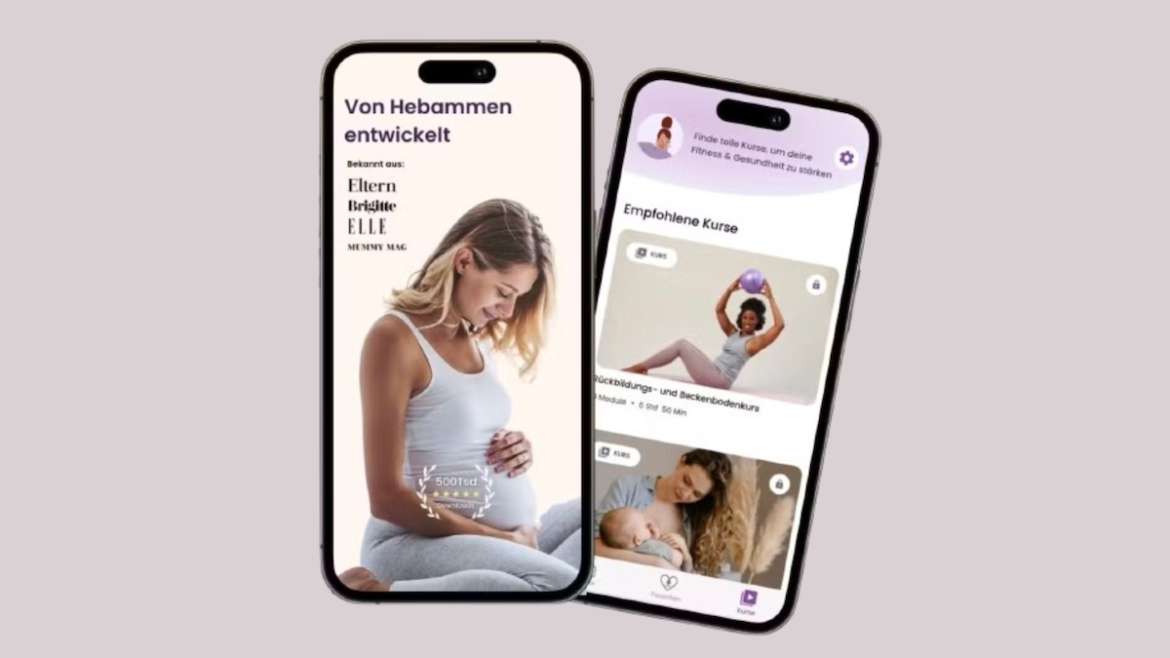
CARÁ Health, a startup from Estonia focused on women’s health and wellness throughout the maternity journey, has raised €620,000 in an early seed funding round. This investment will enable the company to further develop its technology and expand its services into Germany. Founded in early 2022, CARÁ Health has become a vital resource for pregnant women in Estonia. The company’s app, which…