Epstein-Barr Virus (EBV) is a ubiquitous herpesvirus that infects over 90% of the global population. It’s most commonly known for causing infectious mononucleosis (IM), or “mono,” but it has also been implicated in several serious conditions, including various cancers (such as Burkitt’s lymphoma and nasopharyngeal carcinoma), autoimmune diseases (like multiple sclerosis), and post-transplant lymphoproliferative disorder (PTLD). Research into EBV has highlighted potential gender differences in its impact and the immune response it elicits. For instance, studies suggest that women may have a higher risk of developing EBV-associated autoimmune diseases like multiple sclerosis.
In a remarkable breakthrough for the treatment of EBV-driven diseases, Hornet Therapeutics has now emerged from stealth mode, presenting pioneering research published in Science. This marks the first potential drug intervention targeting EBV, a virus that has long eluded effective treatment despite its significant role in various pathologies.
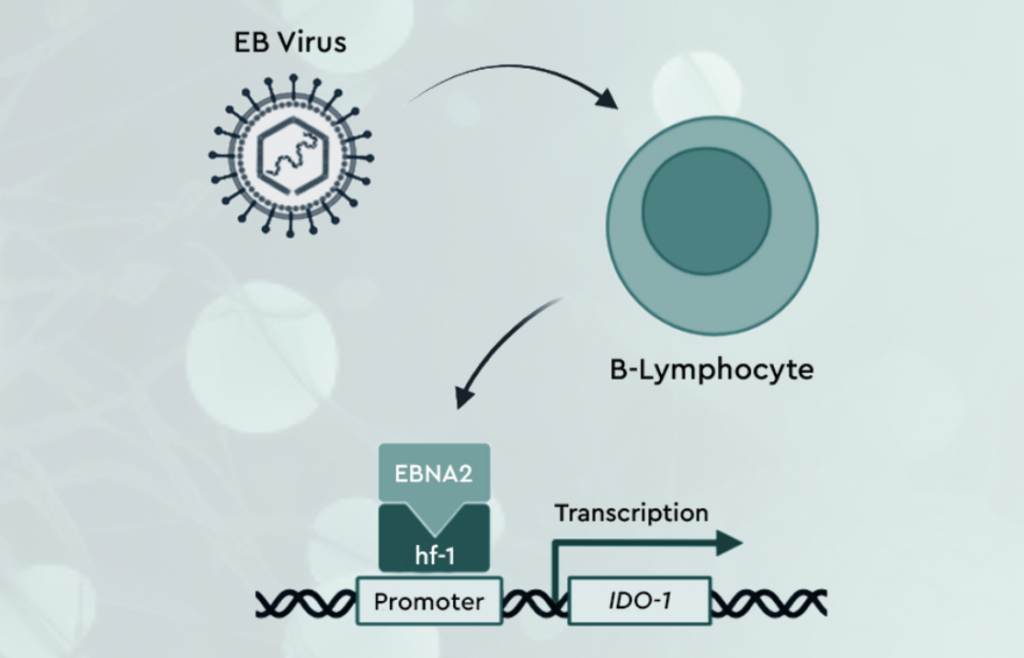
Hornet Therapeutics, incubated by 4BIO Capital, has unveiled HTX-201, the first-ever small molecule drug aimed at treating EBV-driven diseases. This approach targets the host metabolic enzyme IDO-1, which EBV hijacks to establish latency and drive disease progression. By inhibiting IDO-1, HTX-201 disrupts EBV’s ability to maintain infection in B cells, a critical step in the virus’s life cycle. The data published in Science demonstrate that targeting IDO-1 significantly reduces EBV latency, tumorigenesis, and tumor burden in both in vitro and in vivo models. This novel strategy has the potential to prevent EBV-associated diseases, including lymphomas and other malignancies.
Decades of conventional antiviral development have failed to deliver a small molecule compound capable of effectively and specifically targeting EBV. EBV is a major driver of post-transplantation lymphoproliferative diseases (PTLDs) and responsible for a significant proportion of organ loss in solid organ transplant patients, as well as a widely suspected driver of multiple sclerosis (MS). The new findings reported in Science point at IDO-1 inhibition as a potential treatment mechanism for pathologies related to EBV latency.
The use of Hornet’s proprietary, clinically-validated IDO-1 inhibitor, HTX-201, has already demonstrated significant impact on latency, tumorigenesis, tumor burden, and survival in an experimental mouse model of EBV-driven PTLD. The company aims to first develop HTX-201 for the prevention of EBV driven PTLD, where in high-risk solid organ transplant recipients this dangerous complication develops in up to 30% of patients, and the fear of emergence of EBV-driven pathology is ever-present across all transplant populations. By intervening early with HTX-201, Hornet is looking to simplify the management of transplant recipients in the first year post-transplant, when the risk for PTLD and graft loss is highest. With around 80,000 solid organ transplantations in Europe and the US each year, there is a significant unmet need, which Hornet is seeking to address.
Based on the data now published in Science and with access to the clinically tested drug HTX-201, Hornet is poised to progress into phase 1/2 proof-of-concept clinical trials in solid transplant populations within the next 12-18 months. In the future, the company intends to also target other conditions where EBV is implicated, such as MS, infectious mononucleosis, and long-COVID.
Hornet emerges out of stealth with a strategic collaboration and licensing agreement with Kyowa Kirin, enabling Hornet Therapeutics to develop and commercialise HTX-201 (formerly known as KHK2455) in EBV-related diseases. The company has an experienced team of experts in antiviral discovery and commercialisation, led by Dr. Fraser Gray (formerly Vice President, Infectious Diseases, Worldwide Business Development at GlaxoSmithKline) and Professor Christoph Hess (Professor of Medicine at the University of Basel and Director of Experimental Medicine at the University of Cambridge).
Professor Christoph Hess, founder and Chief Scientific Officer at Hornet Therapeutics, added: “In a landmark paper published 60 years ago, Drs. Epstein, Achong and Barr first described EBV – and while we have since built a strong molecular and cellular understanding of the EBV–host interaction, and the propensity of the virus to cause malignancy and promote autoimmunity, we unfortunately still lack an effective and specific treatment for EBV-driven diseases. Our data demonstrate that HTX-201 has the potential to hinder EBV latency in its primary host cells, the B cells. If our findings translate to clinical benefit it would be a huge step forward in this disease area.”
Dr. Fraser Gray, Chief Executive Officer at Hornet Therapeutics, added: “The ability to intervene early with this small molecule inhibitor in multiple settings, including solid transplant populations, will have a significant impact on many patients affected by EBV. We have an experienced development team actively planning the proof-of-concept clinical trials in transplant patients, where we think HTX-201 can address a clear unmet need.”
Dmitry Kuzmin, Managing Partner at 4BIO Capital and Chairman at Hornet Therapeutics, said: “The potential for HTX-201 is significant, not only for transplant patients but in multiple areas where EBV is implicated, such as MS and long-COVID. We are pleased to unveil Hornet with the publication of these groundbreaking data and look forward to continuing to work with the company as it begins proof-of-concept clinical trials in solid organ transplant populations.”