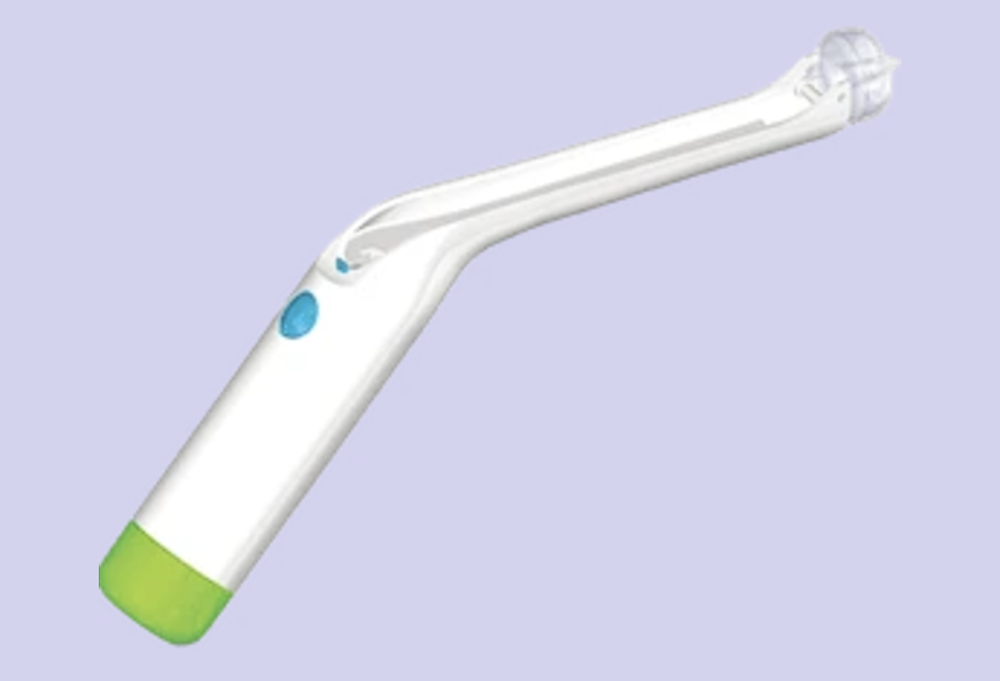
Bioceptive and Trimedic have announced a letter of intent to establish a joint venture aimed at bringing the FDA-approved Cervical Suction Retractor (CSR) to market in Canada and the United States. The device offers an alternative to the traditional tenaculum used in gynecological procedures, including IUD insertion.
The CSR uses gentle suction instead of the pinching mechanism of the tenaculum, which can cause significant pain and may lead women to delay or avoid necessary procedures.
“We are excited to partner with Trimedic to introduce this innovative, safer, and significantly less painful alternative to the tenaculum. This breakthrough technology is designed to improve the experience of women undergoing gynecological procedures and making IUD insertion easier and more comfortable,” said Vicki Briggs, Bioceptive’s Chair of the Board.
“We look forward to working with the team at Bioceptive to bring this groundbreaking women’s health device to market. Together, we are advancing safer, more comfortable solutions for women and redefining standards in gynecological care,” added Tom Ostojic, Managing Partner of Trimedic.
Bioceptive, based at the New Orleans Bioinnovation Center, previously received the Saving Lives at Birth Award in 2013 from USAID, the Bill & Melinda Gates Foundation, and other partners for its IUD insertion system designed for primary care providers and non-physician healthcare workers. Trimedic, founded in 1966, specializes in women’s health and non-hormonal contraception in the Canadian market. The company focuses on introducing solutions in under-represented areas of healthcare, including women’s health and point-of-care diagnostic testing.