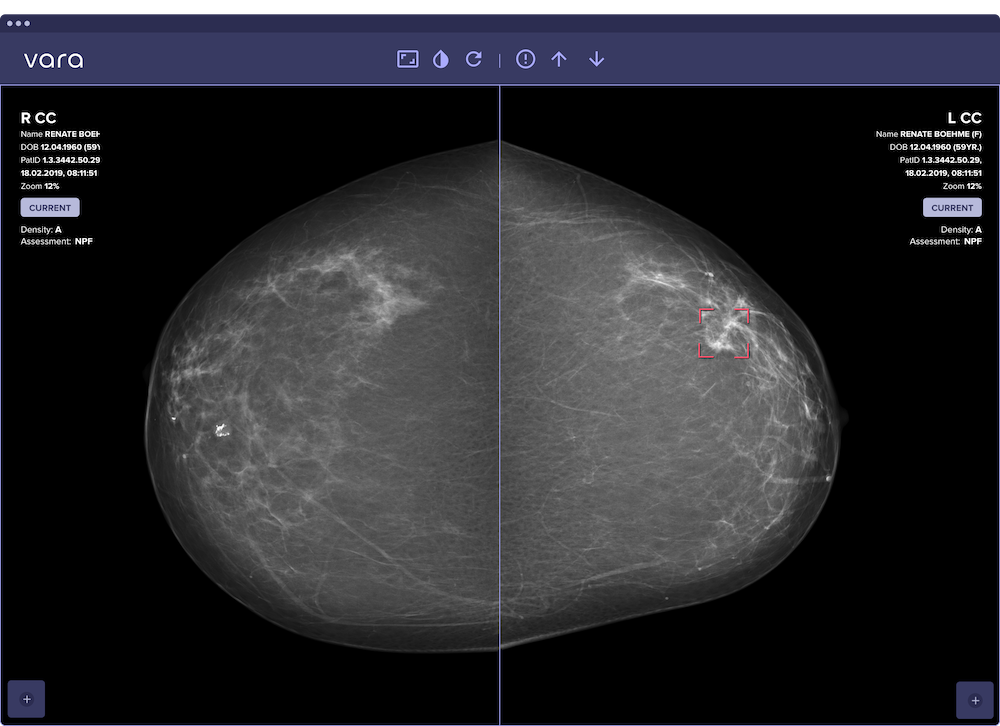
Vara has received a CE certificate for its breast-imaging AI as an independent second reader for screening and diagnostic mammography. The company announced the certification on October 15, 2025.
The CE mark allows the AI to operate as an independent second reader rather than as a decision support tool. The product is available in Europe for screening and diagnostic use, including analysis of mammograms from prior screening rounds. Providers can integrate Vara directly into PACS viewers or through the Vara platform.
Vara currently serves 40% of Germany’s national screening program, processing approximately 150,000 mammograms monthly and more than 1.5 million annually. The company’s PRAIM study, published in Nature Medicine in 2025, enrolled 460,000 women across 12 centers in Germany with no exclusion criteria. The study demonstrated statistically significant improvements in cancer detection rates and workload reduction for radiologists.
“Our ambition has always been to pioneer data-driven AI at a nationwide scale,” said Stefan Bunk, co-founder and CTO of Vara. “The industry has long moved beyond retrospective evidence. Now, by pairing population-wide, real-world results with automatic real-time monitoring, we’re offering a credible, scalable path for AI as an independent reader – and we’re grateful the regulator acknowledged this approach.”
National screening programs in Europe face radiologist shortages, aging populations, and expanded screening age ranges. Vara’s CE mark approval included recognition of the company’s autonomous, prospective AI monitoring system for real-time quality assurance.
Vara is based in Germany and develops AI and cloud workflow tools for breast imaging.



