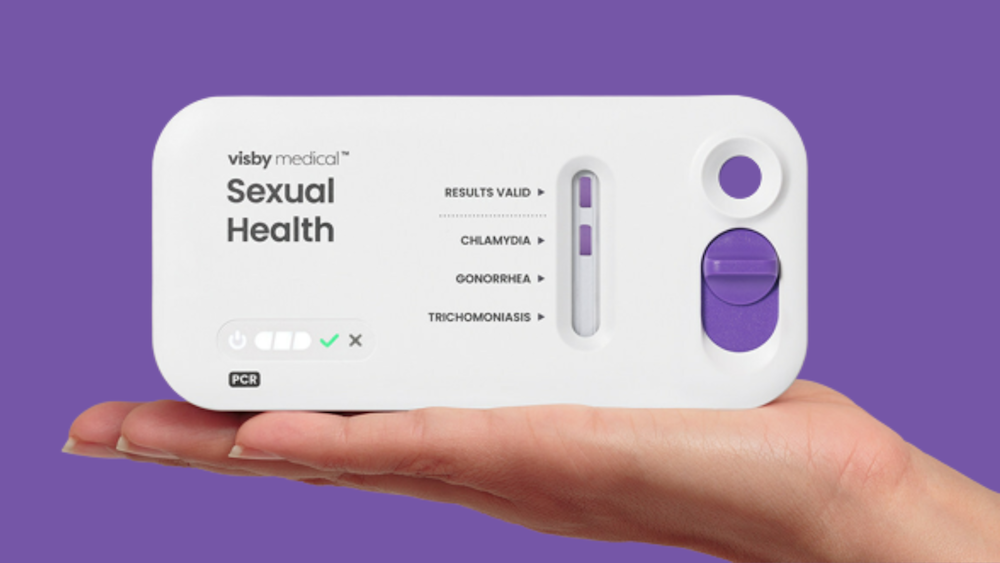
Visby Medical has raised approximately $55 million with potential to reach $65 million in a funding round led by Catalio Capital Management to accelerate launch of its FDA-authorized at-home Women’s Sexual Health Test. The San Jose-based company develps single-use PCR diagnostics that deliver laboratory-accurate results within 30 minutes.
Catalio Capital Management led the round with participation from existing investors including ND Capital, Cedars Sinai Medical Center, Blue Water Life Science Advisors, Pitango Ventures, and John Doerr. The funding supports distribution of Visby’s at-home STI test, which becomes available through direct-to-consumer channels starting July 2025.
“This funding round will enable Visby to deliver on our vision of empowering consumers with reliable and lab-accurate health information from the comfort of their homes, starting with our at-home test for sexually transmitted infections for women,” said Adam de la Zerda, PhD, Founder and CEO of Visby Medical.
The company says its Women’s Sexual Health Test represents the first single-use, disposable PCR diagnostic for at-home use, providing results through a connected smartphone app. Upon receiving positive results, users connect with telemedicine providers for consultation and treatment, addressing healthcare accessibility and privacy concerns in STI testing.
Catalio partner Isaac Ro joins Visby Medical’s board as an observer, while Chuck Alpuche, COO at Imperative Care and former EVP and COO at Insulet Corporation, joins as an independent director. Alpuche brings operational expertise in manufacturing scale-up and cost efficiency as the company expands into consumer markets.
“Visby Medical has delivered the first and only laboratory-grade STI testing solution that can be made directly accessible to individuals,” said Isaac Ro. “We were eager to invest in Visby ahead of their FDA clearance, knowing this would represent a breakthrough moment for the diagnostics industry.”
Visby’s proprietary technology platform delivers PCR results without requiring laboratory instruments, addressing limitations in traditional STI testing that often involves clinic visits, sample collection, and delayed results. The company currently offers FDA-cleared tests for STIs in point-of-care settings and respiratory infections including COVID-19 and influenza.
The at-home STI testing market represents significant opportunity as sexually transmitted infections continue rising, with CDC data showing increases across multiple infection types. Traditional testing barriers including appointment scheduling, clinic access, and result delays create gaps in screening and treatment.
Founded in 2012, Visby Medical positions itself as transforming infectious disease diagnosis through instrument-free PCR technology that fits in users’ hands. The company is expanding its platform with additional tests planned for both at-home and point-of-care markets.



