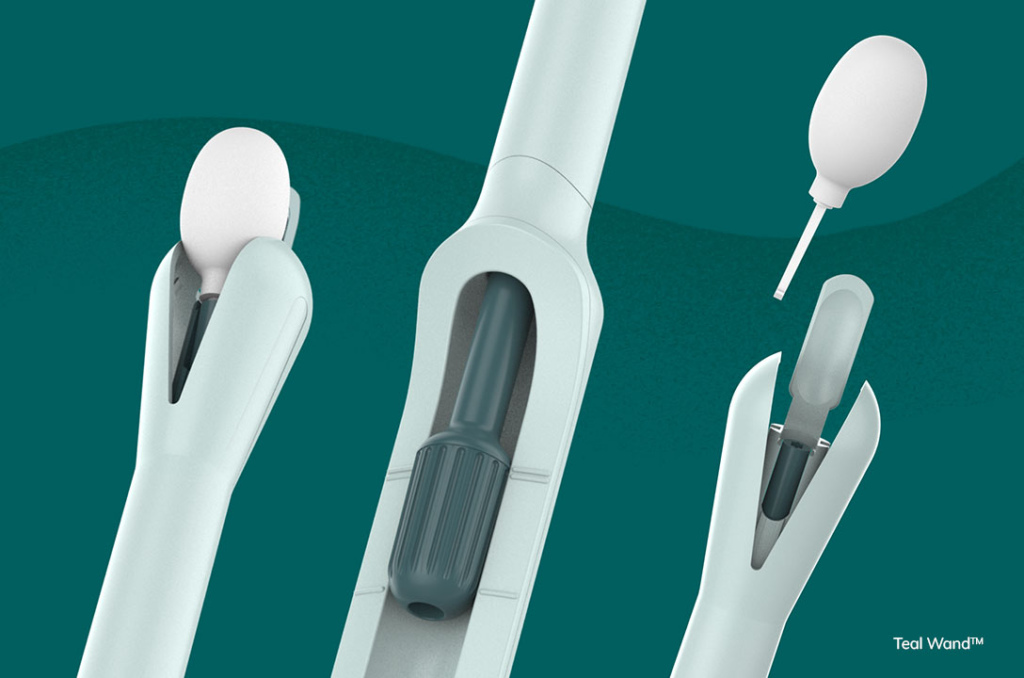
Teal Health, headquartered in San Francisco (USA), has achieved a significant milestone in its mission to improve cervical cancer screening options. The company has been granted Breakthrough Device designation by the US Food and Drug Administration (FDA) for its at-home self-collect cervical cancer screening device, the Teal Wand. This designation follows the successful completion of Teal Health’s clinical trial. The trial surpassed enrollment targets and generated robust clinical performance data, leading to the FDA’s recognition of the device’s potential to enhance diagnosis or treatment of life-threatening diseases.
The Teal Wand enables users to collect their own vaginal sample for cervical cancer screening, either at home or in a health clinic. The collected samples are then sent to a laboratory for testing, with results delivered through the Teal Health patient portal. Designed for ease of use and accessibility, the device caters to diverse body types and health literacy levels.
The effectiveness of the Teal Wand was validated in a study involving over 600 participants from diverse backgrounds. Results showed high user satisfaction, with 97% finding the device easy to use and 94% expressing a preference for self-collection over traditional clinician-based methods.
“I am so grateful to the amazing team at Teal, our hardworking PIs and sites, the supportive participants in our study, and the FDA for recognizing the importance of Teal’s solution to help close the women’s cervical cancer screening gap in the US. The speed of our study shows that if you design for and engage with women to advance women’s health, you’ll be met with resounding enthusiasm from this group that has been overlooked and under researched for far too long. This study and Breakthrough designation is an important moment for women’s health,” comments Kara Egan, CEO and Co-Founder of Teal Health.
Cervical cancer is preventable and is curable 92% of the time, however, it’s the second leading cause of cancer death among women aged 20-39 and incidence is increasing among women aged 30-44. More than half of cervical cancer cases are in people who are not routinely screened, which is in large part due to barriers such as discomfort during the exam, lack of information, time, and access. Studies show that women of all races and socioeconomic levels experience these barriers, with Native American, Black, and Hispanic women resulting in higher underscreened rates. Self-collection will increase access and is a much preferred alternative. It has already been adopted in other countries, including Australia, which increased their screening engagement 50 fold within its first year and is now on track to eliminate cervical cancer as a public health concern by 2035.
“FDA’s recognition of the Teal Wand as a Breakthrough device acknowledges the important public health benefit that self-collection for cervical cancer screening can have on those who are rarely screened or who do not participate in clinician-based screening for cervical cancer. The clinical performance of the Teal Wand shows promise that an at-home self-collection device is possible in the near term and Teal looks forward to working closely with FDA to expedite this option to eligible women and people with a cervix,” states Trena Depel, Vice President of Clinical and Regulatory at Teal Health.