AI-enabled cancer diagnostics company Lunit, recently announced a financial boost with a $150M investment from its shareholders. The South Korean company, listed on the KOSDAQ, plans to invest these funds into new product development and strengthen its foothold in Western markets. The recent funding milestone is complemented by Lunit’s achievement in securing its third 510(k) clearance from the U.S. Food and Drug Administration (FDA).
The deployment of the raised capital is strategically planned: Approximately $52 million is set aside for the development of new AI solutions in cancer diagnostics and treatment. An investment of $38 million is planned for bolstering its subsidiaries in the U.S. and Europe. The company also intends to allocate $30 million for investing in external startups. Additionally, $15 million each is set aside for global talent acquisition and the procurement of patents, licenses, and other intangible assets.
Brandon Suh, CEO of Lunit shared: “We’re deeply grateful for the support and trust from our shareholders. This $150 million capital increase reflects their faith in our mission and technology. We’re eager to use these funds to advance our innovative products, explore new pharmaceutical avenues, and recruit global talent. Our commitment remains steadfast, and we believe that every dollar invested in Lunit can make a significant impact in the fight against cancer.”
Lunit’s increased focus on the U.S. market aligns with the country’s high cancer incidence and mortality rates. In 2020, the U.S. recorded approximately 400 new cancer cases and 144 cancer deaths per 100,000 individuals. The value of cancer diagnostics in the U.S., which stood at $31 billion last year, is anticipated to reach $70 billion by 2032, growing at a CAGR of 8.4%, as per Precedence Research.
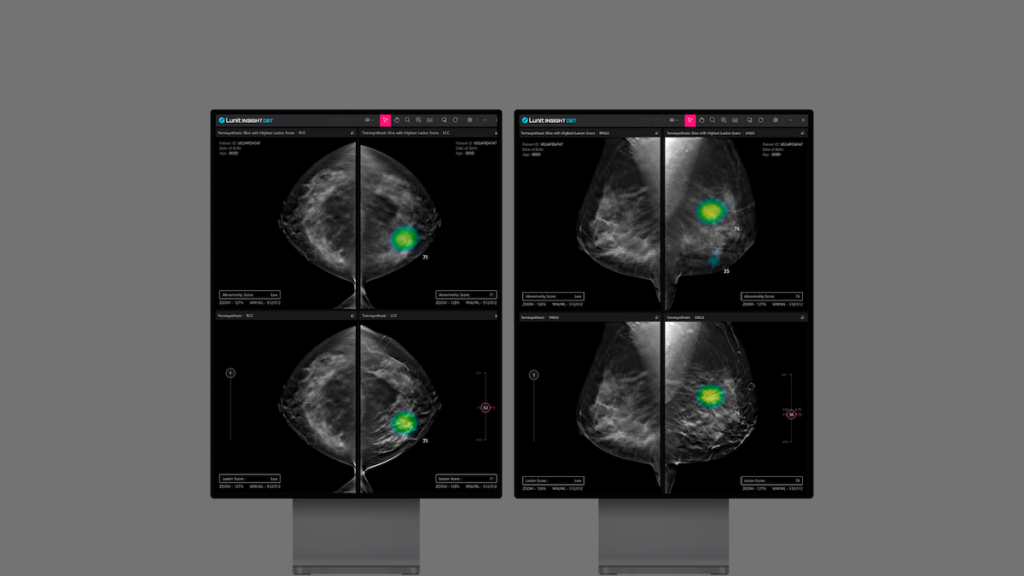
Just a day before the announcement of this significant capital infusion, Lunit revealed that it had received U.S. FDA approval for its AI 3D Breast Tomosynthesis (DBT) solution. This latest approval for Lunit INSIGHT DBT, which automates the analysis of 3D DBT images for breast cancer detection, follows previous clearances obtained in 2021 for Lunit INSIGHT CXR and MMG. The 3D DBT analysis solution has also received CE marking under the latest Medical Device Regulation as of March.
Recently, Lunit participated in the inaugural project of the White House’s CancerX initiative, showcasing innovative solutions in cancer screening, diagnosis, treatment, and survivorship care. The company is a founding member of this initiative.



