Femasys has achieved a significant milestone with the first regulatory approval worldwide for its FemBloc delivery system, a non-surgical approach to female permanent birth control. The company announced today that it has received CE mark certification under European Union Medical Device Regulation (EU MDR).
The FemBloc system represents a paradigm shift in contraceptive technology as the first-ever non-surgical alternative to tubal ligation, which has remained largely unchanged since its introduction in the 1800s.
For the complementary FemBloc blended polymer component, Femasys has completed an expedited G12 Special MDR Audit for Class III devices, with the Notified Body recommending CE mark approval pending final European Medical Agency (EMA) review. Full approval is expected by mid-2025.
“European approval for the FemBloc delivery system was achieved through interactive collaboration with the Notified Body to accelerate the approval of this innovative technology in the first region of the world. This is a significant step towards a long-awaited turning point for women seeking a safer, more accessible permanent contraceptive option as an alternative to the centuries-old surgical approach, the sole option available today,” stated Kathy Lee-Sepsick, Femasys’ CEO and Founder.
This approval positions Femasys to disrupt the global permanent contraception market by addressing significant barriers to access. Traditional surgical sterilization carries risks including infection, bleeding, injury to nearby organs, anesthesia-related complications, and even death. The procedure also excludes women who may not qualify as good surgical candidates due to obesity or medical comorbidities.
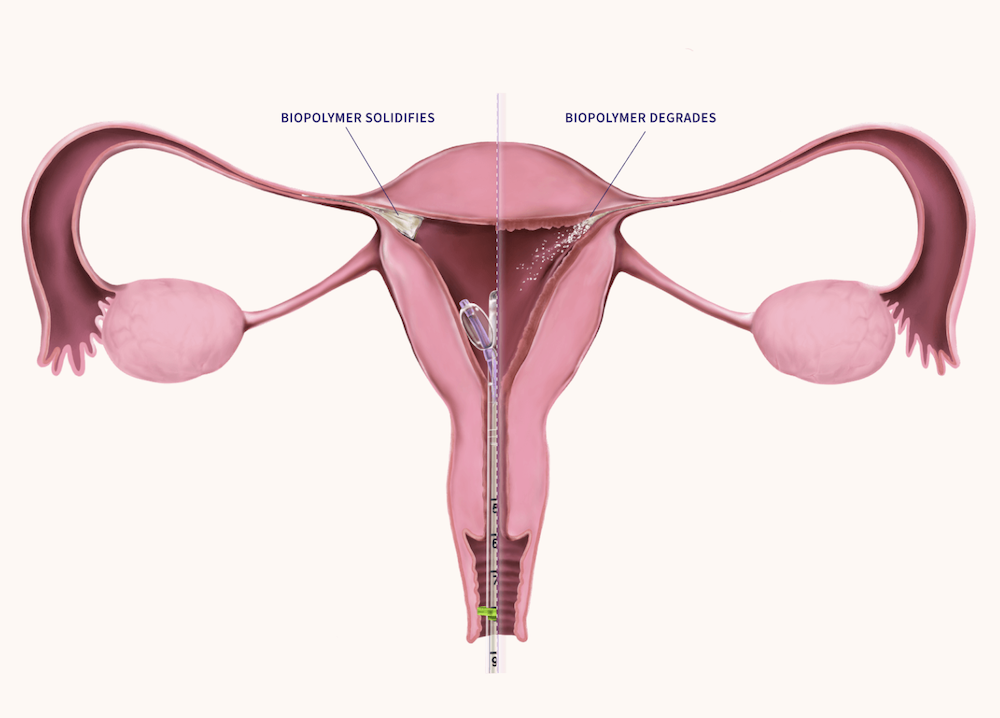
FemBloc’s approach involves minimally-invasive placement of a proprietary synthetic tissue adhesive (blended polymer) into both fallopian tubes simultaneously. The polymer degrades over time, producing nonfunctional scar tissue that permanently blocks the tubes. This procedure can be performed in-office, potentially offering reduced risks and substantially lower costs.
The company is preparing for a potential market launch in select European countries while simultaneously continuing enrollment in its FDA IDE approved FINALE pivotal clinical trial (NCT05977751) for U.S. regulatory approval.
This regulatory milestone aligns with Femasys’ broader strategy of developing accessible, in-office solutions for women’s health. The company has built a diverse product portfolio including:
- FemaSeed Intratubal Insemination – a first-line infertility treatment with FDA clearance and approvals in Europe, UK, Canada and Israel
- FemVue – a companion diagnostic for fallopian tube assessment
- FemCerv – a tissue sampler for cervical cancer diagnosis
- FemCath and FemChec – diagnostic products for FemBloc’s ultrasound-based confirmation test



