Femasys has received its first European commercial order worth approximately $400,000 for FemBloc Permanent Birth Control in Spain. The order marks the company’s entry into Europe following regulatory approval earlier this year for the non-surgical permanent contraceptive.
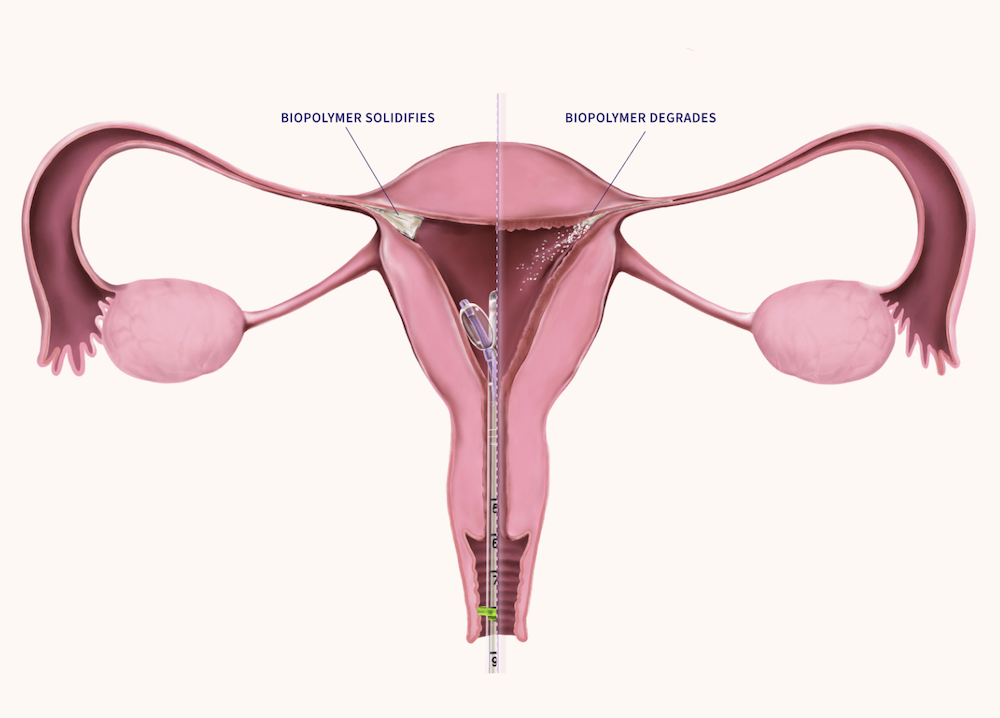
FemBloc uses a patented delivery system to place a proprietary polymer into both fallopian tubes, which degrades and forms natural scar tissue for permanent occlusion. The procedure is performed in-office without surgery, eliminating risks associated with anesthesia, infection, and recovery downtime compared to surgical sterilization.
“We are proud to be executing on our mission to expand access to FemBloc, an innovative, non-surgical solution and the only advancement in permanent birth control that offers a less invasive alternative to current and formerly available methods,” said Kathy Lee-Sepsick, Chief Executive Officer and Founder of Femasys.
The company positions FemBloc as addressing an unmet need in women’s reproductive health, with no comparable non-surgical alternatives currently available on the market. Published clinical trial data demonstrated effectiveness, five-year safety, and high patient and practitioner satisfaction.
Femasys is simultaneously conducting the FINALE pivotal trial for U.S. FDA approval, with enrollment ongoing. The company also develops fertility products including FemaSeed intratubal insemination and FemVue diagnostic imaging.
Spain becomes the first country globally to offer access to FemBloc through distribution partners Comercial Medico Quirúrgica and Durgalab.



