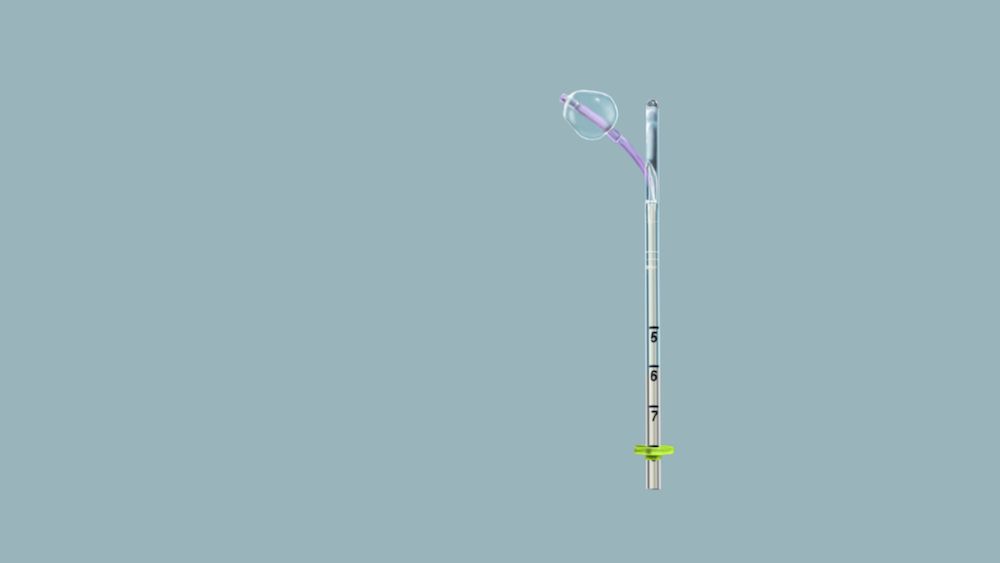
Femasys, a biomedical company aiming to meet women’s needs worldwide by developing a suite of product candidates that include minimally invasive, in-office technologies for reproductive health, has announced that its product, FemCath, the first FDA-cleared intrauterine catheter for selective tubal evaluation, is now commercially available. FemCath is used in conjunction with Femasys’ FemVue device for an ultrasound-based diagnostic test as part of an infertility evaluation, which is essential prior to any infertility treatment, including with Femasys’ other biomedical solution in development, FemaSeed.
FemCath utilizes Femasys’ proprietary delivery platform, which involves placement of balloon technology close to the opening of a selected fallopian tube for directed delivery. Femasys’ other biomedical solutions in development, FemaSeed and FemBloc, utilize the same delivery platform for directed delivery of other materials. In the case of FemaSeed, sperm is delivered directly to the tube where conception occurs and for FemBloc, a proprietary biopolymer is delivered to both tubes for nonsurgical permanent birth control.
“This is one more important milestone reached in 2022 as we continue to expand much needed options for women with our suite of innovative products,” said Kathy Lee-Sepsick, founder, president and chief executive officer of Femasys. “We are encouraged to improve the options available for those struggling with infertility and expect if FemaSeed is approved to bring a solution to the front-end of care. In the meantime, FemCath will provide another measure of incremental revenue for Femasys when used with FemVue for any conditions requiring tubal evaluation.”



