Femasys, a biomedical company specializing in women’s reproductive health, recently revealed that it has secured an Investigational Device Exemption (IDE) from the US Food and Drug Administration (FDA). This exemption allows Femasys to conduct a clinical trial, the so-called “FINALE” trial, aimed at evaluating the safety and efficacy of their revolutionary product, FemBloc.
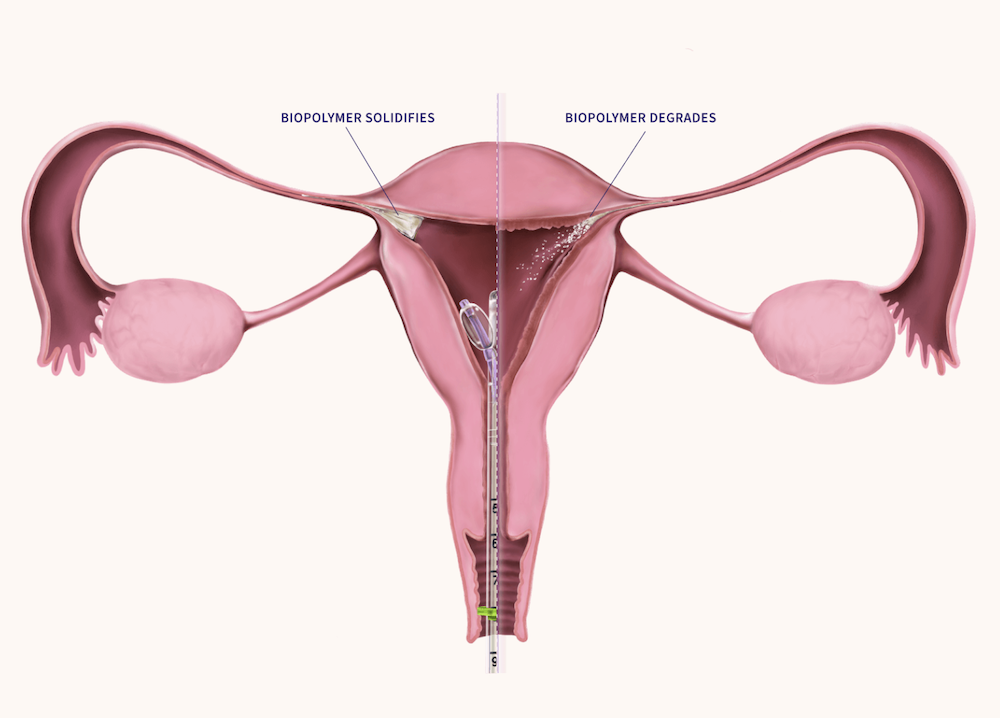
FemBloc is designed to provide a safer, more accessible option for permanent birth control, without the need for anesthesia, incisions, or permanent implants. The solution features Femasys’ proprietary delivery platform, which places balloon technology close to the opening of both fallopian tubes. This in-office approach is designed to eliminate the risks of incisions, anesthesia, and hormones. As a nonsurgical procedure that is implant free, FemBloc delivers a biopolymer that is expected to expel within 3 months.
FemBloc and FemaSeed, a localized directional insemination solution for infertility, are the company’s two lead product candidates. Once approved, these therapeutic solutions will supplement Femasys’ existing diagnostic products, which include FemVue for ultrasound-assisted fallopian tube assessment, FemCath, an intrauterine catheter for selective fallopian tube evaluation, and FemCerv, a endocervical sampler for cervical cancer diagnosis.
“On this one-year anniversary of the overturn of Roe v. Wade, the need for more accessible birth control options for women has never been more important,” stated Kathy Lee-Sepsick, founder, president, and CEO of Femasys. “FemBloc has been designed to be a solution that provides an alternative to surgical tubal ligation, reducing unnecessary risks and substantial cost associated with surgery. In addition, FemBloc could provide an option for women who have been using temporary birth control methods long term that may require implants or hormones.”
The FINALE (Prospective Multi-Center Trial for FemBloc INtratubal Occlusion for TranscervicAL PErmanent Birth Control) pivotal trial, set to kick-off in the third quarter of 2023, aims to evaluate the safety and efficacy of this novel, non-surgical approach to female permanent birth control. The trial design is a prospective, multi-center, open-label, single-arm study. The primary endpoint will be the pregnancy rate, to be analyzed once 401 women have used FemBloc for one year. Before enrolling the remaining subjects, the company plans to enrol 50 women to primarily gather preliminary safety data. Interim analysis is planned once 300 women have used FemBloc for one year, with follow-ups continuing annually for five years post-market.



