Shanghai-based Yipurun Biotech (YPR) has announced the approval of its Corina Intrauterine Drug-Eluting System (“Corina”) in China. This device is set to advance the treatment of patients with moderate-to-severe intrauterine adhesions (IUA) who undergo transcervical resection of adhesions (TCRA). The approval was granted based on a randomized controlled clinical trial that demonstrated Corina’s superior efficacy compared to the current standard-of-care treatments.
Intrauterine adhesions (IUA) are scar tissues that form within the endometrial cavity, often leading to reduced menstrual flow, amenorrhea, infertility, and recurrent miscarriage. The condition poses a significant threat to the reproductive health of women of childbearing age, particularly in China, where the incidence rate of IUA is alarmingly high, affecting up to 25% of women who have undergone prior abortion or curettage procedures.
Standard treatment for IUA, TCRA, involves surgically separating the adhesions to restore the uterine cavity’s anatomical shape. However, despite this intervention, the recurrence rate of IUA remains as high as 62.5%, and the pregnancy success rate post-surgery is disappointingly low, ranging from 22.5% to 33.3%. While various adjuvant therapies have been developed to improve outcomes, their efficacy has been limited.
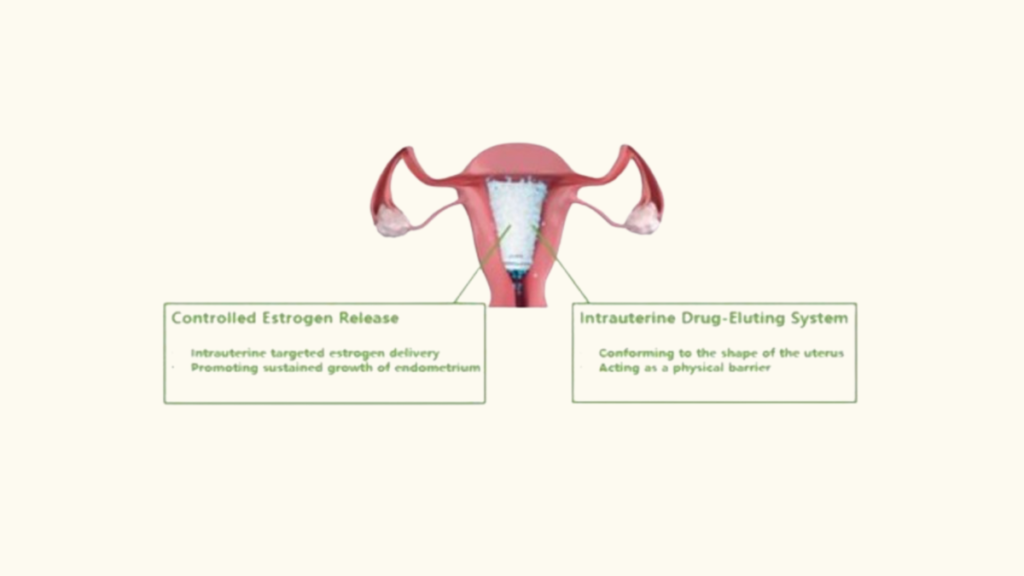
Corina is the world’s first intrauterine drug-eluting system specifically designed to address the challenges associated with IUA treatment, according to YPR. Made from non-degradable, medical-grade material and carrying the active ingredient estradiol, Corina is engineered to conform to the unique shape of the female uterus. Upon insertion, it acts as a physical barrier to prevent re-adhesion and enables targeted delivery of estradiol directly to the endometrium.
Using YPR’s proprietary controlled-release technology, Corina can continuously release estradiol for up to 60 days post-procedure. This sustained release is crucial for promoting endometrial growth and reducing the risk of re-adhesion, offering a significant improvement over standard therapies.
The registration study for Corina demonstrated statistically significant and clinically meaningful reductions in intrauterine adhesion at 60 days post-procedure compared to standard care. Additionally, Corina showed a notable improvement in endometrial thickness from baseline, further highlighting its potential to enhance reproductive outcomes for women with IUA.
YPR is a wholly-owned subsidiary of Puyi Biotech, a company dedicated to developing innovative, implantable therapeutics across various medical fields, including women’s health. Since its founding in 2012, Puyi Biotech has leveraged its expertise in biomedical material science and drug-eluting technology to address unmet clinical needs. The company has attracted investments from investors like Goldman Sachs Alternatives, Legend Capital, and Northern Light VC.



