
Amplexd Therapeutics has secured $2 million in funding from an Asia-based family office specializing in life sciences. This investment supports the finalization of product development and the initiation of Phase 1/2 clinical trials for non-invasive treatments targeting Cervical Intraepithelial Neoplasia (CIN).
“The funding marks a major milestone in our R&D efforts for our two therapies after extremely promising preclinical studies were completed. The capital will enable us to finalize development and IND submissions ahead of first-in-human clinical trials, expected to commence later this year,” said Amplexd Therapeutics Co-Founder and CEO Alia Rahman.
Amplexd Therapeutics, a clinical-stage patient-scientist-led biotech company, specializes in developing accessible treatments for HPV-induced CIN. The recent funding will enable the company to finalize product development and submit Investigational New Drug (IND) applications, with clinical trials expected to commence later this year. These therapies aim to provide low-cost, patient-friendly alternatives to the current “watch and wait” approach for low-grade CIN and invasive surgeries for high-grade CIN.
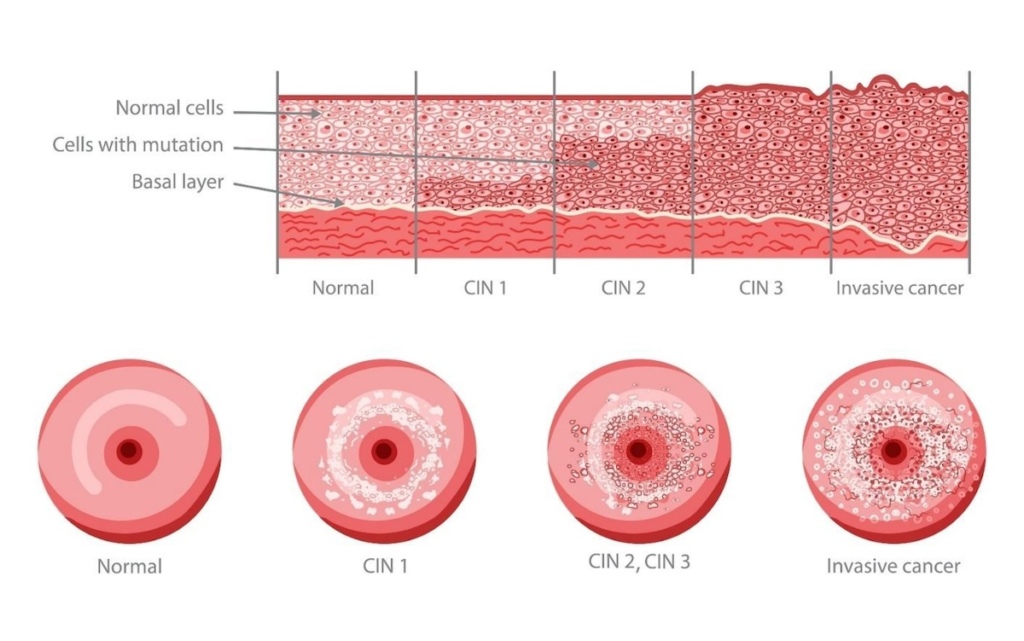
The company was founded in 2022 by CEO Alia Rahman, whose personal experience with chronic CIN following a diagnosis of carcinoma in situ (high-grade CIN) and subsequent invasive surgery inspired her to develop non-invasive treatment options. Amplexd’s therapies utilize a primary active ingredient, Epigallocatechin gallate (EGCG), a polyphenol derived from green tea, which has shown efficacy against CIN in research settings but had not been developed as a commercial therapeutic. Rahman’s goal was to create a non-invasive topical therapy to avoid the psychological burden and trauma associated with chronic CIN and invasive surgeries.
Amplexd’s first product is an intravaginal suppository designed for low-grade CIN, while the second product is a photodynamic therapy (PDT) system for high-grade CIN. Both treatments are designed to selectively target neoplastic cells, potentially sparing patients from invasive surgeries and making treatment more accessible, particularly in low and middle-income regions.
Cervical cancer, largely preventable when treated at the CIN stage, is the fourth leading cause of cancer deaths in women worldwide. CIN, resulting from certain oncogenic varieties of HPV, can transform cervical cells into invasive cancer if left untreated. About five percent of cervical screenings in the US and Europe reveal abnormalities, with significantly higher rates in Asia, Latin America, and Africa. Amplexd’s innovative therapies aim to address this significant public health issue.
The funding positions Amplexd to make a substantial impact on women’s health globally. As the company prepares for clinical trials, its non-invasive treatments hold the potential to reduce the burden of cervical cancer, offering hope for millions of women.



