
French women’s health company Womed recently announced significant advancements in the treatment of intrauterine adhesions (IUAs), a major cause of female infertility, with its product, Womed Leaf. The announcement came following the successful results of the PREG2 clinical trial, which were shared by Prof. Jean-Louis Benifla during the Choix des Armes conference in Marseille, France and suggest that Womed’s technology can help women struggling with IUAs, which are often a consequence of uterine surgeries and can lead to infertility, recurrent miscarriages, and pain.
IUAs result from the abnormal scarring of the uterus, leading to the pathological binding of its walls. The condition can occur in up to 45% of women undergoing procedures like dilation and curettage or fibroid removal. The traditional treatment for IUAs, adhesiolysis (surgically cutting the adhesion), faces a high recurrence rate, leaving many women uncertain about their fertility futures.
Womed Leaf is a mechanical barrier specifically designed to prevent IUAs. Made from a proprietary polymer, this soft film is inserted similarly to an intrauterine device (IUD) at the end of a uterine procedure. It then expands to cover the entire cavity, preventing the uterine walls from contacting each other for a week before being naturally expelled.
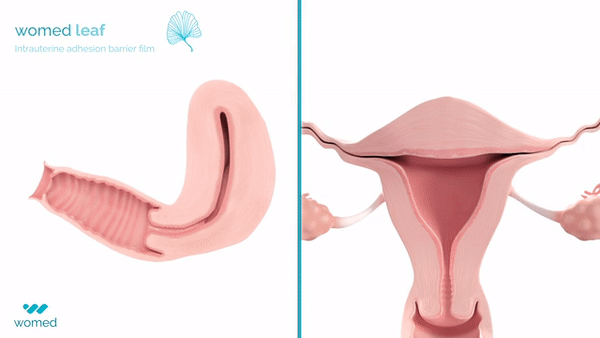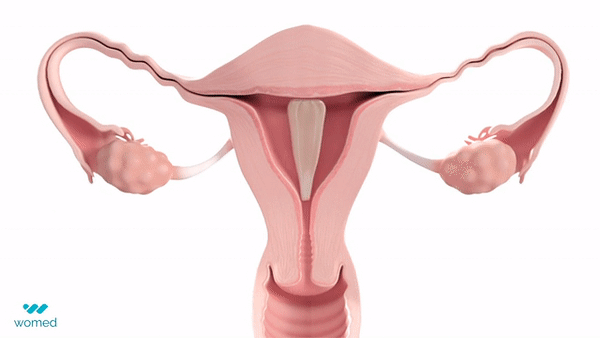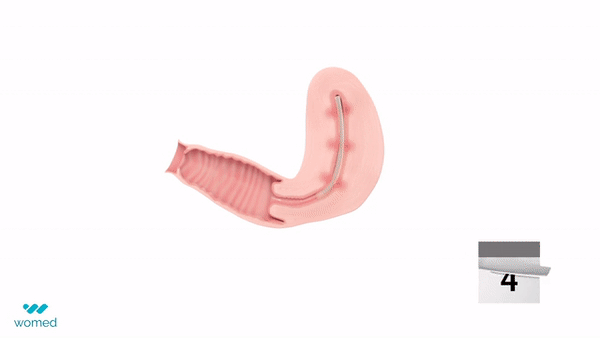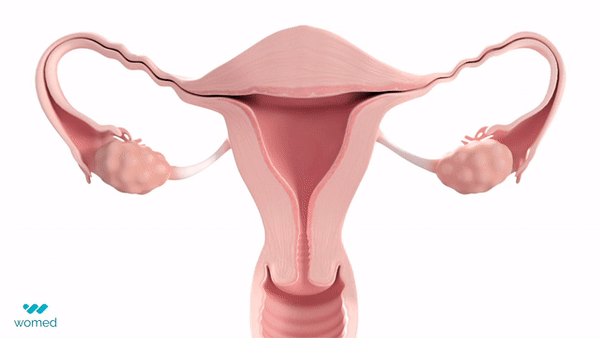
The PREG2 trial, an international, randomized clinical study, involved 160 women with severe or moderate IUAs who were either assigned to receive Womed Leaf or not post-adhesiolysis surgery. The findings revealed that, at a six-week follow-up, women treated with Womed Leaf were 2.4 times more likely to have no adhesion compared to those in the control group, alongside favorable outcomes across all other efficacy endpoints. Importantly, no serious or device-related adverse events were reported, affirming the safety of Womed Leaf.
“The results of this landmark study, rigorously conducted in several hospitals around the world, are great news for our patients,” said Prof. Herve Fernandez, Bicetre University Hospital – APHP, Paris, France and principal investigator of the study. “Womed Leaf, which protects the cavity during the healing phase, is breaking new ground and becomes, to the best of our knowledge, the first technology to achieve a proof of efficacy in this highly complex population.”
“Womed has achieved an unprecedented feat and we are very proud to be the first team to offer a solution to women with scarred uterus in their exhausting journey to become pregnant,” said Gonzague Issenmann, co-founder and CEO of Womed. “Womed Leaf, which is already registered in Europe and Brazil, will be marketed through commercial partners.”



