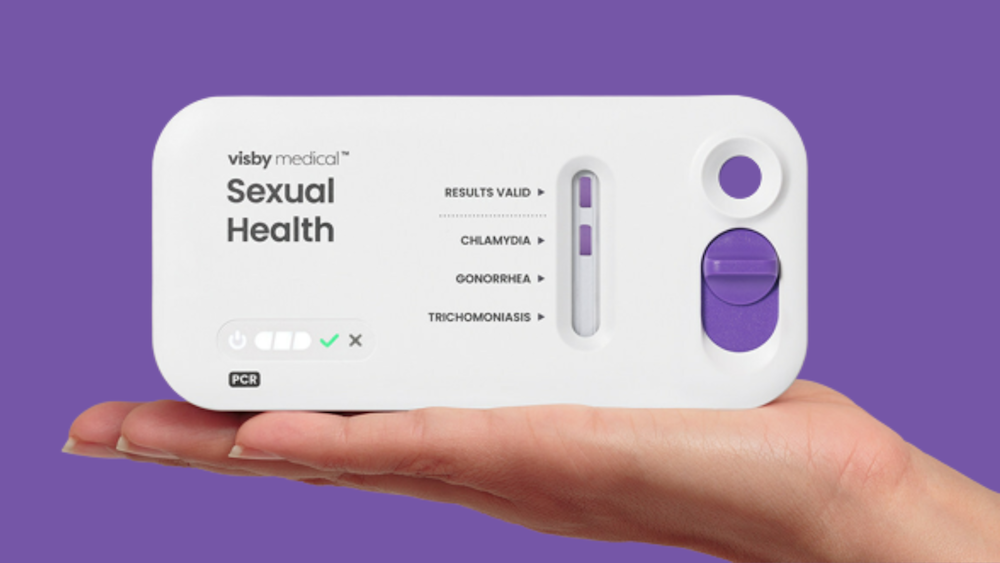
Visby Medical has received De Novo authorization from the U.S. Food and Drug Administration (FDA) for its Women’s Sexual Health Test for Over the Counter (OTC) use. According to the company it is the first-ever PCR (polymerase chain reaction) diagnostic device approved by the FDA for OTC home use for any indication.
The test allows women to test reliably, rapidly, and privately at home for Chlamydia, Gonorrhea, and Trichomoniasis – the three most common curable sexually transmitted infections (STIs). Unlike other home-based STI tests on the market that require mailing samples to a laboratory, Visby’s technology provides results directly to users within 30 minutes using PCR technology, widely recognized as the gold standard in diagnostic accuracy.
“This approval is not just a milestone for Visby Medical but marks a transformative moment in medical diagnostics,” said Adam de la Zerda, PhD, Founder and CEO of Visby Medical. “We’ve achieved something incredible; our palm-sized, single-use PCR test is simple to use and replaces a bulky, large, expensive laboratory instrument. After 12 years of development, our device delivers rapid, reliable results directly into the hands of consumers, with unparalleled convenience and privacy. We also built a state-of-the-art, fully automated manufacturing line ready to rapidly scale production in anticipation of growing consumer demand.”
The technology has undergone extensive clinical validation, with studies involving over 2,000 lay users demonstrating that the Visby Medical Women’s Sexual Health Test delivers accuracy comparable to traditional laboratory-based PCR machines. This level of accuracy enables healthcare providers to confidently prescribe treatment based on test results. The system includes an intuitive companion app that guides users through the entire testing process—from sample collection to test execution and result interpretation—while providing a seamless connection to further care options.
“The clinical significance of bringing a rapid, highly accurate PCR diagnostic test into the home environment cannot be overstated,” said Gary Schoolnik, MD, Chief Medical Officer of Visby Medical. “Extensive clinical studies validate that this test empowers women to quickly understand what steps to take next, giving them the privacy, control, and confidence to seek the care they need. Importantly, many patients infected with these STIs are non-symptomatic, yet they can still suffer serious long-term health consequences. Our test directly addresses this silent epidemic by enabling detection and treatment.”



