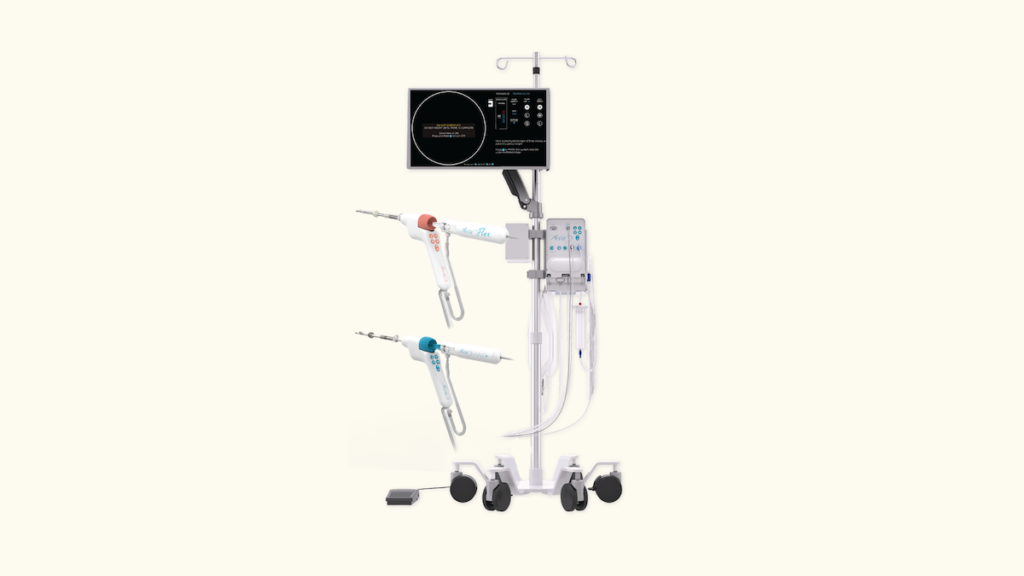
Meditrina, a developer of gynecologic medical devices, announces the CE Mark and UKCA Mark approval for its Aveta Hysteroscopy System. This approval, aligned with Regulation (EU) 2017/745, marks a significant achievement for Meditrina, allowing the company to expand its international footprint and further its commitment to advancing women’s health worldwide.
The Aveta Hysteroscopy System features a fully disposable 4.6mm resecting hysteroscope, designed for the efficient removal of polyps and fibroids. This advanced technology, coupled with its ergonomic design, enables healthcare professionals to conduct minimally invasive procedures with enhanced visualization and control.
“We are pleased to receive the UKCA and CE Mark approvals, which affirm the quality and performance of our Aveta Hysteroscopy System,” stated Csaba Truckai, CEO of Meditrina. “Our initial international procedures in Spain and Denmark highlight our commitment to improving women’s healthcare globally. We are dedicated to collaborating with healthcare providers worldwide to deliver innovative solutions that empower women to lead healthier lives.”



