When Dr. Saad Slimani was completing his radiology residency at Casablanca University Hospital, he noticed something counterintuitive: The obstetric ward kept referring pregnant patients to his department for fetal ultrasounds, even though obstetrics was their specialty. The radiology team could barely keep up with demand, and patients sometimes waited weeks for scans, leading to delayed diagnoses.
That bottleneck is the core problem DeepEcho set out to solve. Founded in 2020, the Moroccan AI healthtech company has since achieved FDA 510(k) clearance for its fetal ultrasound analysis platform, published research in Nature Communications, and expanded operations across three continents.
From Casablanca to FDA Clearance
DeepEcho emerged when Youssef Bouyakhf, whose father had established one of Morocco’s first AI labs, connected with Slimani through a mutual friend. Bouyakhf had been exploring how AI could improve maternal health outcomes. Slimani and his colleague Dr. Leïla Noureddine, also a radiologist in Casablanca, had witnessed firsthand how sonographer shortages affected patient care.
During early research, the team discovered the problem wasn’t unique to resource-constrained settings – the US market faces similar challenges.
Rather than targeting complex diagnostic edge cases, they focused on fetal biometry – the foundational measurements of fetal growth that every prenatal ultrasound requires. Their literature review revealed surprisingly little research applying modern deep learning to automate it.
The co-founders spent roughly a year manually annotating thousands of ultrasound images, then designed a prospective, multi-site clinical study to validate their AI models – the first of its kind in Africa. Their findings, published in Nature Communications in November 2023, showed DeepEcho’s models performing with consistency that matched or exceeded human sonographers. By June 2025, the company had secured FDA 510(k) clearance.
A Crisis on Two Continents
In 2023, 669 women died of maternal causes in the United States, with Black women facing mortality rates nearly three times higher than white women. Over 35% of US counties are now classified as maternity care deserts, affecting more than 2.3 million women of reproductive age.
“When you examine the data from the United States – particularly the elevated rates among Black women – it’s notable that the wealthiest nation on earth faces similar structural problems to those we see in the Global South,” says Slimani, co-founder and Chief Medical Officer.
The company now operates with a dual market strategy. In Morocco, DeepEcho has partnered with the Ministry of Health to deploy across several regions, with discussions underway in Kenya and Nigeria. In the US, the company is piloting with maternal-fetal medicine specialists in New York.
“In Morocco, we’re running clinical and impact studies to demonstrate reduced mortality and lower costs for health systems,” explains CEO Bouyakhf. “In the US, the focus is on efficiency – helping MFM specialists increase patient throughput without compromising quality.”
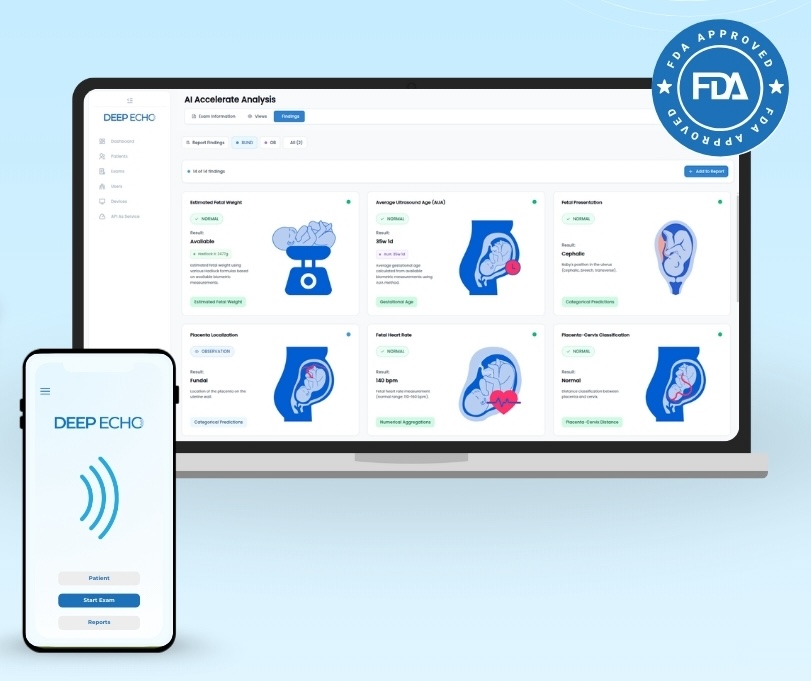
One Platform, Multiple Applications
DeepEcho’s platform guides users through 18 standard planes of fetal ultrasound assessment, but the clinical value varies by user expertise. For trained sonographers, it’s an efficiency tool that automates caliper placement and plane identification. For healthcare workers with limited training, it provides real-time guidance that enables them to perform scans they couldn’t otherwise attempt.
“For an experienced sonographer, they’ll review the final segmentation and move on,” Slimani notes. “For a midwife in a rural area, our solution provides the confidence to perform the scan in the first place.”
The company has also developed a “blind sweep” approach for minimally trained operators, who can run the probe across the patient’s abdomen while the AI extracts diagnostic information. With handheld ultrasound devices now priced below premium smartphones, DeepEcho sees an opportunity to move fetal imaging outside hospitals entirely.
Beyond Biometry
While fetal biometry earned DeepEcho its FDA clearance, the company’s research extends further. Dr. Noureddine is leading studies into AI-detected biomarkers for preeclampsia, gestational diabetes, postpartum hemorrhage, and anemia.
“Our research indicates there are markers on standard B-mode ultrasound that can differentiate between preeclamptic and non-preeclamptic patients – markers not visible to the human eye,” explains Noureddine, who leads clinical operations while maintaining her radiology practice.
If validated, a simple ultrasound sweep could identify pregnancy risks that currently require additional testing or go undetected entirely.
Building a Global Company from Africa
DeepEcho has raised $2.2 million to date from Plug and Play, UM6P Ventures, Al Mada Ventures, and Algebra Ventures.
“Building deep tech in a country not traditionally known for it created an interesting dynamic,” recalls Bouyakhf. “Local investors suggested we target US funds, while US investors saw us back then as too early-stage and locally focused. UM6P Ventures ultimately backed us, followed by Plug and Play, Al Mada Ventures and Algebra Ventures which allowed us to prove our technology through clinical studies, publications, and FDA clearance.”
The current fundraise focuses on market access and deployment, with the company positioning itself for partnerships that could unlock deployment at scale.



