
In clinics across the world, the speculum, a fundamental tool for women’s healthcare, has remained largely unchanged since its development in the 19th century. For Hege Hansen, a midwife with over 20 years of clinical experience, and Dr. Stine Andreasen, a gynecologist and PhD in Obstetrics, this lack of innovation in essential medical tools – from speculums to labor induction catheters – became the catalyst for founding Induvita, a medtech company developing three distinct devices to modernize women’s healthcare equipment.
“Through our clinical experience, we identified significant gaps in women’s health equipment – both for patients and healthcare providers,” explains Hansen. “The current standard of care relies on tools that weren’t designed for their intended purpose, yet have been accepted due to lack of alternatives.”
This observation is starkly illustrated by current labor induction practices. About 30% of pregnancies in Western European countries require induction, yet the standard tool is a urinary catheter designed for men. “Currently, 97% of procedures in Norway use a urinary catheter that isn’t CE marked for this purpose,” Dr. Andreasen notes. “We’re developing purpose-built equipment that considers both clinical efficacy and patient comfort.”
Founded in August 2022, Norway-based Induvita has rapidly developed three complementary innovations: an improved induction catheter, a redesigned speculum, and a medical training simulator. The speculum features modified sidewalls to better visualize the cervix in pregnant women and comes in different sizes for varying patient needs. Their training simulator, already in use at Norwegian universities and hospitals, provides crucial hands-on experience for medical professionals before patient interaction.
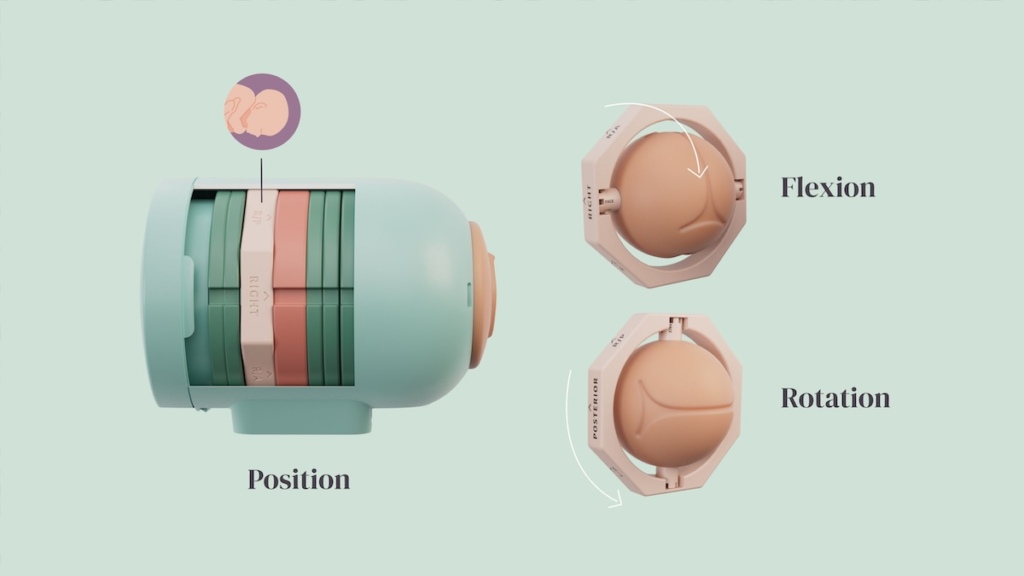



“Our goal is to create medical devices that enhance both clinical outcomes and patient experience,” says Dr. Andreasen. “This includes considering factors such as material choice, ergonomic design, and varying patient anatomies.”
Induvita has made remarkable progress in just over two years. The company has secured 6.3 million NOK ($550K USD) from investors, plus additional non-dilutive funding from Innovation Norway and the Norwegian Research Council. Their training simulator is already generating revenue, providing early market validation for their approach.
The company is now preparing for broader market entry, with CE marking for their speculum expected by August/September 2025. Clinical trials for the induction catheter will follow, with the company targeting an 18-24 month timeline for full regulatory approval. Market research is also already underway in Nordic countries, with plans to expand into broader European markets and eventually the US.
“We’ve maintained a highly efficient development process by focusing on essential innovations that address clear clinical needs,” says Hansen, highlighting the company’s strategy of working with specialized consultants and designers while maintaining a lean core team.
Looking ahead, Induvita is preparing to raise additional funding in March/April 2025 to support their regulatory approvals and market expansion. The company sees opportunities not just in labor and delivery, but in improving everyday gynecological care, particularly for underserved patient populations – like for example young women – requiring different specifications.
By bringing comprehensive innovation to women’s healthcare equipment, Induvita aims to establish new standards in both clinical efficacy and patient comfort. “Our mission extends beyond creating new devices,” concludes Dr. Andreasen. “We’re working to establish a new standard of care that prioritizes both medical outcomes and patient dignity.”



