Femasys (Nasdaq: FEMY) has received CE mark certification for its complete FemBloc system, marking the first global regulatory approval for a non-surgical permanent birth control solution. The US-based company can now market FemBloc throughout the European Economic Area, including 27 EU member states.
The European Medicines Agency reviewed the Class III FemBloc blended polymer component before granting CE mark certification under EU Medical Device Regulation. This approval follows the March 2025 CE mark for the delivery system component, completing regulatory clearance for the entire system.
“European approval of the entire FemBloc System represents a major milestone for Femasys and the field of women’s health,” said Kathy Lee-Sepsick, CEO and Founder of Femasys. “It marks the first global regulatory endorsement of our non-surgical permanent birth control solution.”
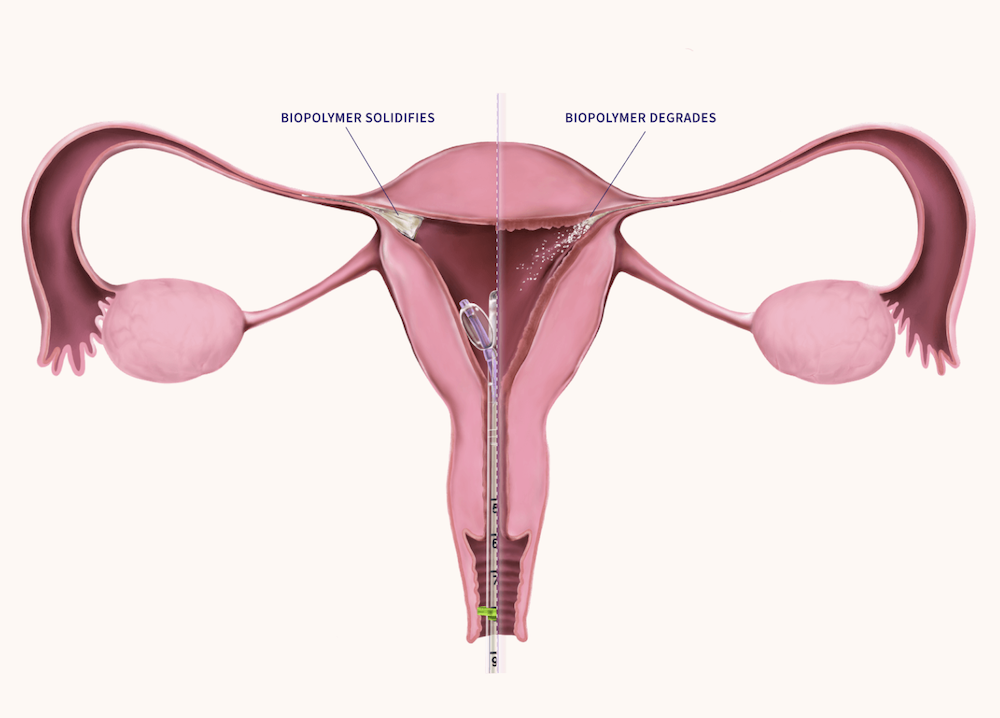
FemBloc provides an in-office alternative to surgical sterilization by placing a proprietary blended polymer into both fallopian tubes through a patented delivery system. The polymer safely degrades and forms natural scar tissue to permanently block the tubes, eliminating risks associated with anesthesia, infection, and recovery downtime.
The technology addresses limitations of current permanent birth control options, which typically require surgical procedures with associated risks and recovery periods. FemBloc’s office-based approach aims to make permanent contraception more accessible and cost-effective.
Femasys plans to begin European commercialization in Spain through existing distribution partnerships before expanding to additional countries. The company continues enrolling participants in its FDA-approved FINALE pivotal trial for U.S. market approval.
Published clinical trial data demonstrates effectiveness, five-year safety, and high patient satisfaction rates. The system includes FemChec, a diagnostic product enabling ultrasound-based confirmation of procedure success.
The approval represents an advancement in permanent contraception options, providing women with a non-surgical alternative that eliminates traditional barriers associated with surgical sterilization procedures.



