Escala Medical has completed a $4.5 million funding round to accelerate global expansion of its FDA-approved medical device for pelvic organ prolapse. The round included the EIC Fund following a €2.5 million grant awarded through the EIC Accelerator program in October 2023, along with private investors from the United States and Israel.
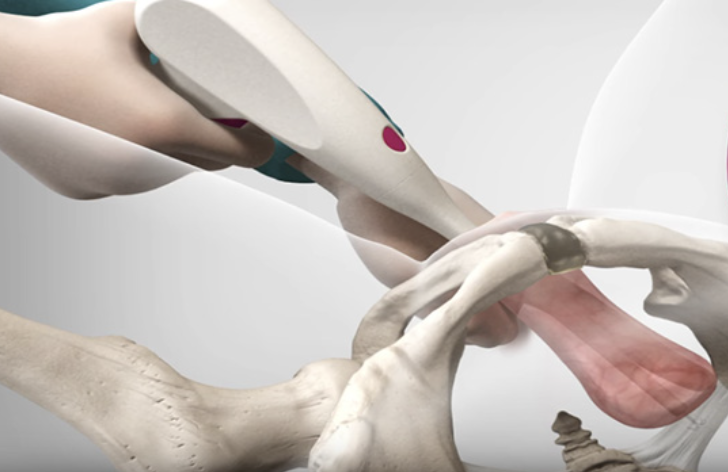
The Israeli medical technology company develops treatments for women with pelvic organ prolapse and launched its flagship device commercially in the U.S. in 2023. Hundreds of procedures have been performed using the device, which enables treatment in physicians’ offices rather than hospital settings.
“This funding marks an important milestone as we continue to scale our operations and bring our technology to more women worldwide,” said Dr. Edit Goldberg, CEO of Escala. “We remain committed to advancing the next generation of our Mendit device to improve outcomes and expand access to millions of women worldwide who suffer from organ prolapse.”
The funding will support expansion of Escala’s U.S. commercial operations while preparing for European market entry pending CE approval. The company has also signed a distribution agreement with a Singapore-based partner covering Southeast Asia markets.
Pelvic organ prolapse affects millions of women globally, occurring when pelvic floor muscles weaken and organs shift from their normal position. Traditional treatment options often require surgical procedures in hospital settings, creating barriers to access and increasing healthcare costs.
“Being selected for the EIC Accelerator and receiving both a grant and equity investment from the EIC Fund is a major vote of confidence in our technology, our team, and our vision,” said Robert D. Auerbach, M.D., Chair of Escala.



