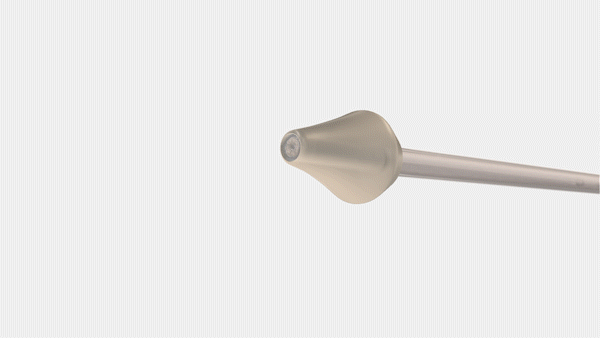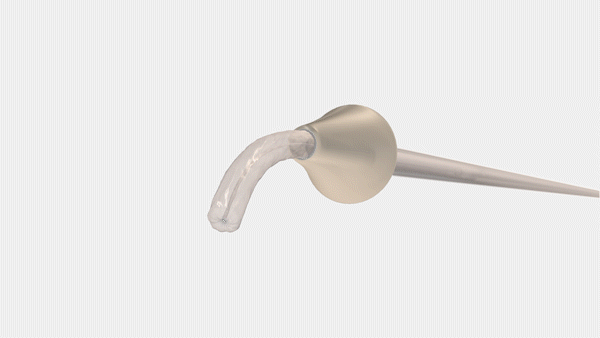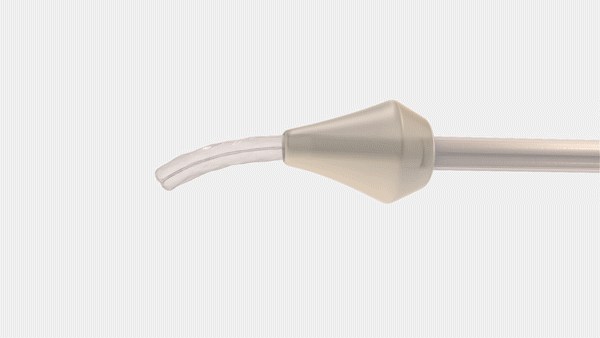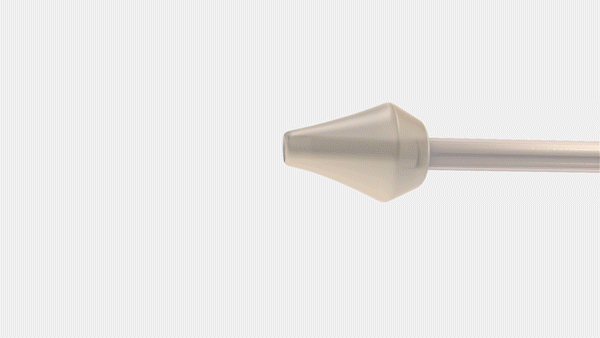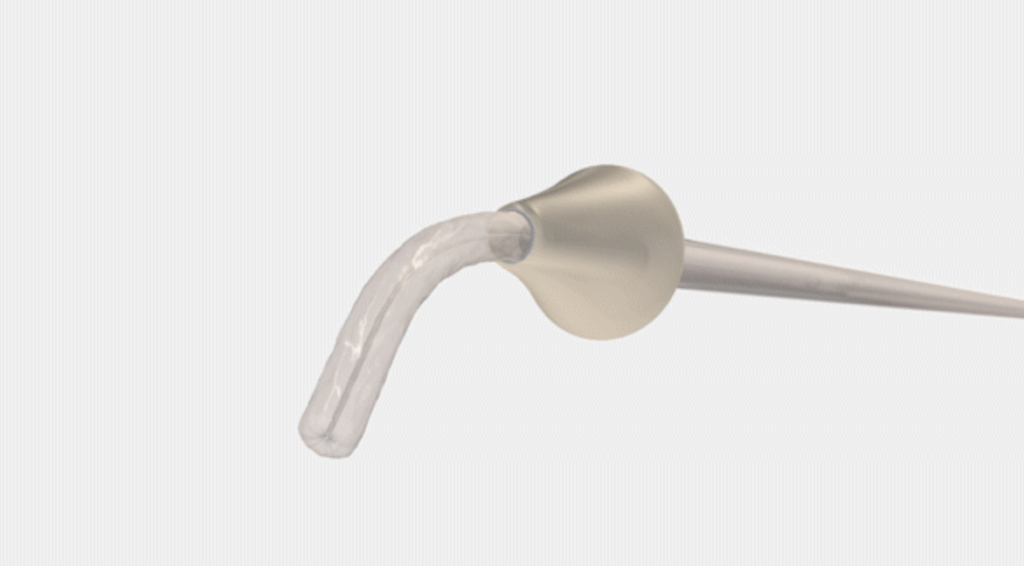
Bayer and CrossBay Medical have announced a development and licensing agreement to create a single-handed inserter for intrauterine systems (IUS), integrating CrossBay’s CrossGlide technology with Bayer’s hormonal IUD portfolio.
One of the challenges in IUD insertion is the pain and discomfort experienced by some women. The process, which involves inserting a small T-shaped device into the uterus, can cause pain and cramping, varying in intensity from woman to woman. This discomfort is often due to the dilation of the cervix and the placement of the IUD.
The new inserter, using CrossGlide technology, aims to potentially improve the comfort of intrauterine placement, and might reduce discomfort during the insertion process. This could also shorten the procedure time required by healthcare professionals.
“As a leader in Women’s Healthcare, we are committed to further drive innovation for women worldwide that addresses their unmet medical needs,” said Juergen Eckhardt, M.D., Member of the Executive Committee of Bayer’s Pharmaceuticals Division and Head of Business Development, Licensing & Open Innovation. “Partnering with CrossBay Medical has the potential to offer women an opportunity to consider the full range of contraceptive options available.”
“We invented CrossGlide to help improve the insertion experience by creating a flexible system that follows the shape of a woman’s cervix. By doing this, we created a new paradigm for office-based procedures for both women and healthcare providers,” said Piush Vidyarthi, founder and CEO of CrossBay Medical“We are partnering with Bayer as a market leader in intrauterine contraception as we believe more women will be better served using the CrossGlide technology and therefore provided one of the most effective birth control options for consideration.”
The agreement also involves adapting the manufacturing process for the CrossGlide technology, incorporating Bayer’s manufacturing expertise, particularly at its production site in Turku, Finland.