
Amara Therapeutics, an Irish medtech company focused on women’s health, has announced the launch of a clinical trial to evaluate its digital therapeutic solution for overactive bladder (OAB) in women. The company has also recently secured a €1.8 million funding round backed by Enterprise Ireland, bringing its total funding to €4.8 million since founding in 2021.
Overactive bladder affects millions of women worldwide, causing symptoms like urinary urgency, frequency, nighttime urination (nocturia), and urgency urinary incontinence (UUI). These symptoms impact quality of life, while healthcare barriers including fragmented networks, specialist shortages, and rising costs limit access to effective treatment.
Brendan Staunton, co-founder and CEO of Amara Therapeutics, stated: “This is a huge milestone for Amara Therapeutics and takes us one step closer to revolutionizing the treatment of OAB globally. Access to high-quality, affordable care is increasingly out of reach for millions of women who suffer from OAB. Our mission is to ensure every patient has access to affordable, clinically proven behavioral therapy.”
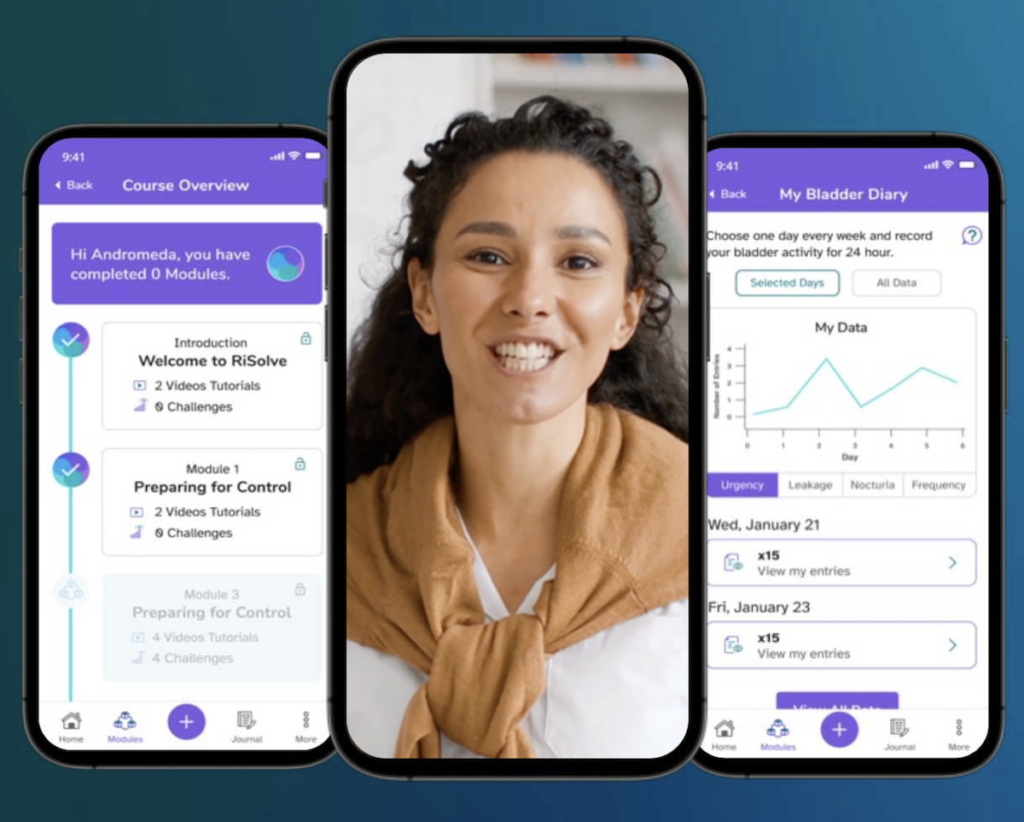
The randomized controlled trial, named “The APPROVE Trial,” will evaluate the efficacy and safety of RiSolve, Amara’s prescription digital therapeutic (PDTx). The study will compare the RiSolve App versus standard behavioral education in women with OAB symptoms, measuring symptom reduction, quality of life improvements, and treatment satisfaction.
Nearly 600 participants will be enrolled across 10 centers throughout the United States, with recruitment conducted virtually. Medstar Health, an academic health system comprising ten hospitals and over 300 care locations across Maryland, Virginia, and Washington, D.C., is sponsoring the trial.
“We are thrilled to enroll the first participants and are excited to lead this pioneering study to evaluate the potential of prescription digital therapeutics in treating conditions like overactive bladder,” said Dr. Alexis Dieter, director of research for Urogynecology and Reconstructive Pelvic Surgery at MedStar Health and Principal Investigator for the clinical trial. “By leveraging digital health solutions, we aim to be able to offer women a novel, non-invasive OAB therapy that they can access from the comfort of their own home.”
RiSolve is an app-based digital therapeutic designed to provide behavioral therapy and cognitive behavioral therapy (CBT) to adult women diagnosed with OAB. Developed over the past two years, the prescription digital therapeutic is indicated for an 8-week treatment period to reduce OAB symptoms through digital interventions.
The APPROVE Trial is backed by The Foundation for Female Health Awareness, a nonprofit organization focused on research and treatment options for female pelvic floor conditions.
“Our mission is to support innovative research that enhances the well-being of women,” said Dr. Mickey Karram, Co-Founder and Chief Executive Officer of the foundation. “RiSolve represents a promising new avenue in digital medicine, and we look forward to the insights this trial will provide.”
The recent funding will enable Amara Therapeutics to complete this clinical trial and commercialize its digital platform. Founded in 2021 by Brendan Staunton, Dr. Emma Carr, and Geoffrey Cundiff as a spinout from the University of Galway, the company develops app-based solutions for pelvic and bladder health conditions.



