Organon has announced the completion of its acquisition of Dermavant Sciences from Roivant, a deal first reported in September 2024. The completion comes at a strategic time, as the FDA reviews VTAMA cream’s potential expansion into atopic dermatitis treatment, with a decision expected in Q4 2024.
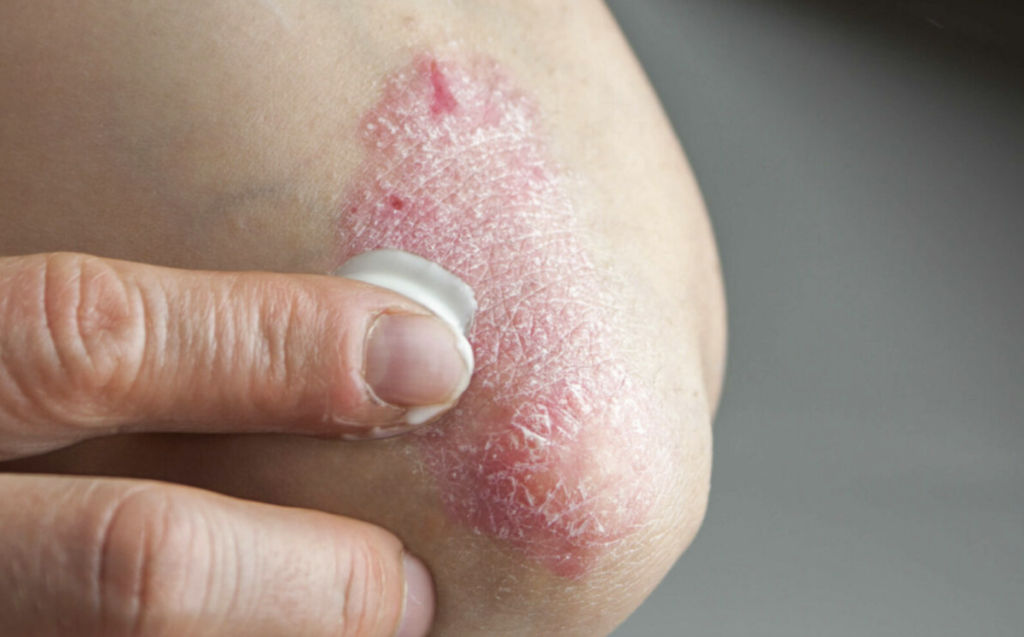
VTAMA (tapinarof) cream, 1%, Dermavant’s flagship product, represents a unique addition to Organon’s portfolio as a nonbiologic, non-steroidal topical therapy. The product is currently FDA-approved for treating mild, moderate, and severe plaque psoriasis in adults, notably with no safety label warnings or precautions and without restrictions on location and duration of use or body surface area.
“The future of dermatology depends on innovative treatments like VTAMA, and Organon’s acquisition of Dermavant allows us to further expand our existing portfolio of established brands and biosimilar dermatology treatments,” said Kevin Ali, Chief Executive Officer of Organon. “Integrating the expertise of Dermavant into Organon’s U.S. organization marks the beginning of a new chapter in dermatology. We are excited to bring this nonbiologic non-steroidal topical option to the millions of patients suffering from a chronic skin condition like plaque psoriasis and, potentially in the future, atopic dermatitis.”
The timing is particularly significant as atopic dermatitis, commonly known as eczema, has been associated with a higher disease burden for women compared to men. This aligns with Organon’s mission to improve women’s health outcomes, extending their reach into conditions that disproportionately affect women.
“I would like to thank Kevin and the entire Organon team for their partnership in the acquisition of Dermavant,” said Mayukh Sukhatme, MD, President and Chief Investment Officer of Roivant. “This deal represents a true win-win outcome for Organon and Roivant in our mutual goal to address patient needs and is emblematic of Roivant’s ability to form non-traditional, value-enhancing collaborations on important medicines. We believe that Organon’s strong global commercial footprint will maximize the impact of VTAMA for patients globally, and we are excited to continue to share meaningfully in the success of VTAMA along the way.”



