Organon has announced its plans to acquire Dermavant Sciences, a Roivant company focused on innovative immuno-dermatology treatments. The deal, which is expected to be finalized in the fourth quarter of 2024, will allow Organon to expand its dermatology offerings in the U.S. market, particularly with Dermavant’s flagship product, VTAMA (tapinarof) cream, 1%.
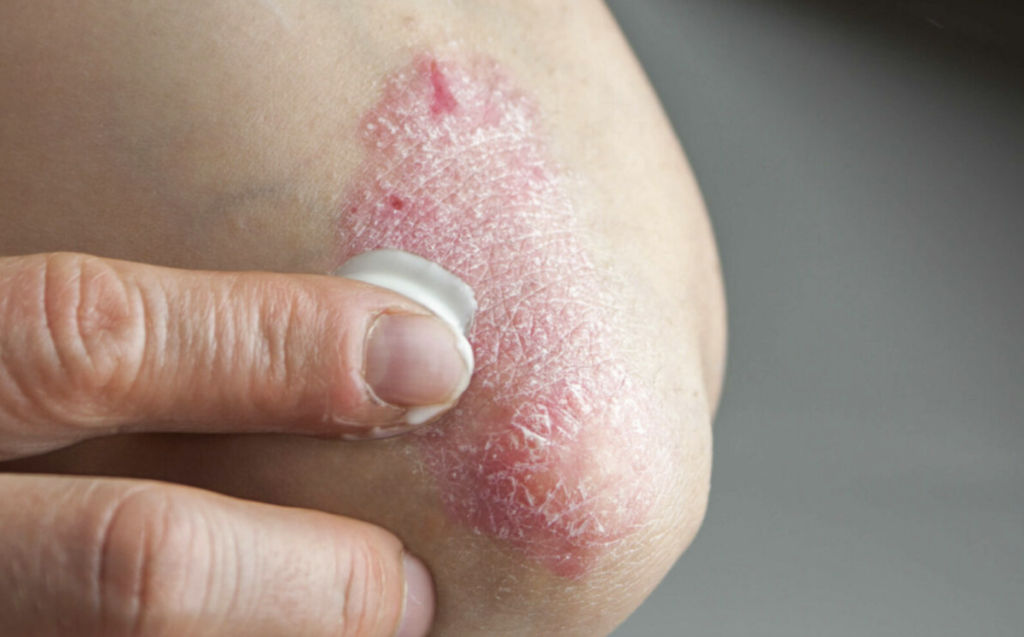
VTAMA cream, a novel, non-steroidal topical therapy, was approved by the FDA in May 2022 for the treatment of plaque psoriasis in adults. It is currently under review by the FDA for an additional indication to treat atopic dermatitis in both adults and children aged two years and older, with a decision expected by the end of 2024. The acquisition will mark Organon’s first significant venture into the U.S. dermatology space, complementing its existing global dermatology operations.
Organon CEO Kevin Ali highlighted the strategic significance of the acquisition, stating, “We look forward to combining Dermavant’s strong dermatology presence in the U.S. with Organon’s regulatory expertise and global market reach. This will allow us to bring VTAMA cream, a patient-focused dermatological innovation, to millions suffering from plaque psoriasis and potentially atopic dermatitis.”
Psoriasis is a common chronic skin disease that affects more than 8 million adults in the U.S. alone, with an estimated 125 million people affected globally. Atopic dermatitis, another widespread skin condition, impacts over 16 million adults and nearly 10 million children in the U.S., with women disproportionately affected by the disease burden. The acquisition aligns with Organon’s mission to improve healthcare for women, particularly in areas where conditions affect them differently, like in dermatology.
Under the terms of the agreement, Organon will acquire Dermavant for a total consideration of up to $1.2 billion. This includes an upfront payment of $175 million and a milestone payment of $75 million, contingent on FDA approval of VTAMA for atopic dermatitis. The deal also includes potential commercial milestone payments of up to $950 million. Additionally, Organon will pay tiered royalties on net sales of VTAMA, with the rights to the product outside of China but excluding Japan.
VTAMA has already made significant strides in the dermatology market, quickly becoming the leading branded topical treatment for plaque psoriasis within just two months of its launch. Dermavant CEO Todd Zavodnick shared, “This is an unparalleled opportunity for Dermavant to continue our mission to improve dermatology care. Organon’s global scale will allow us to maximize the potential of VTAMA cream and reach even more patients.”



