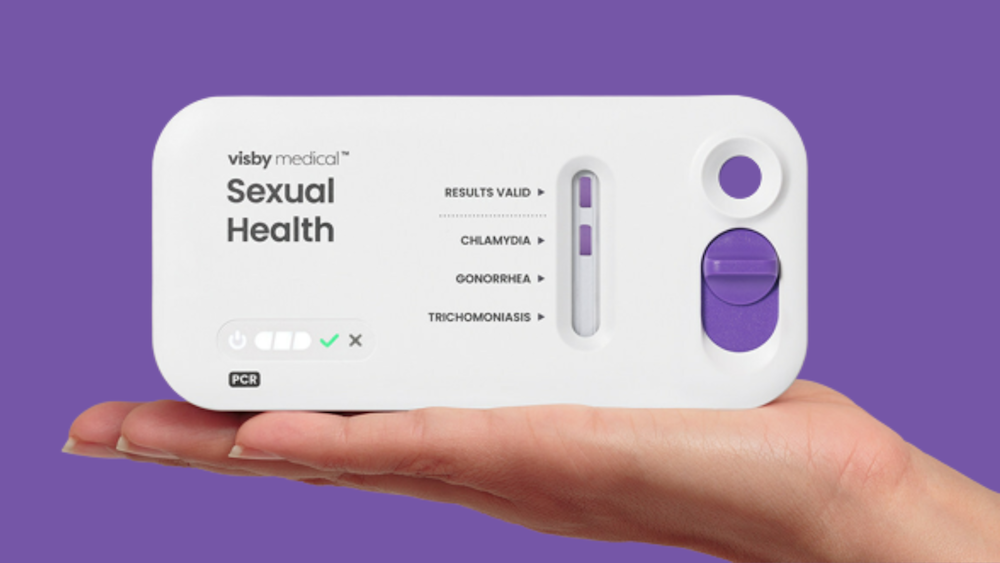
On Tuesday, Visby Medical announced that its second-generation point-of-care sexual health test has been granted 510(k) clearance and a CLIA waiver by the US Food and Drug Administration. The test, which utilizes PCR technology, can detect sexually transmitted infections caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis in women and can provide results in less than 30 minutes with approximately 97 percent accuracy, according to the company.
Compared to the original device that received FDA clearance in 2021, the new version of the test has improvements in workflow, manufacturability, and reliability.
Visby Senior VP of Operations Mark Medlen explains: “We are excited that the clearance of the second generation Sexual Health Test will enable us to move production to our fully automated lines, allowing us to deliver more tests to eagerly waiting customers There is a significant unmet need to improve accuracy and time-to-diagnosis in STI testing, which often takes two to five days or more to return results. In April 2022, the Centers for Disease Control and Prevention reported that STIs have reached an all-time high for the sixth consecutive year, with a nearly 30% increase in gonorrhea and chlamydia between 2015 and 2019.”
Visby Chief Medical Officer Gary Schoolnik added: “Providing more customers with access to the Visby platform will enable clinicians to make informed treatment decisions during the span of a single clinic visit. This will help reduce overtreatment and undertreatment rates, as well as the number of patients who are lost to care. By providing a prompt and accurate test result, the use of this test will lower the likelihood that an infected patient will develop pelvic inflammatory disease. It will expedite the treatment of that patient’s sexual partner, and it will reduce the spread of sexually transmitted infections overall.”



