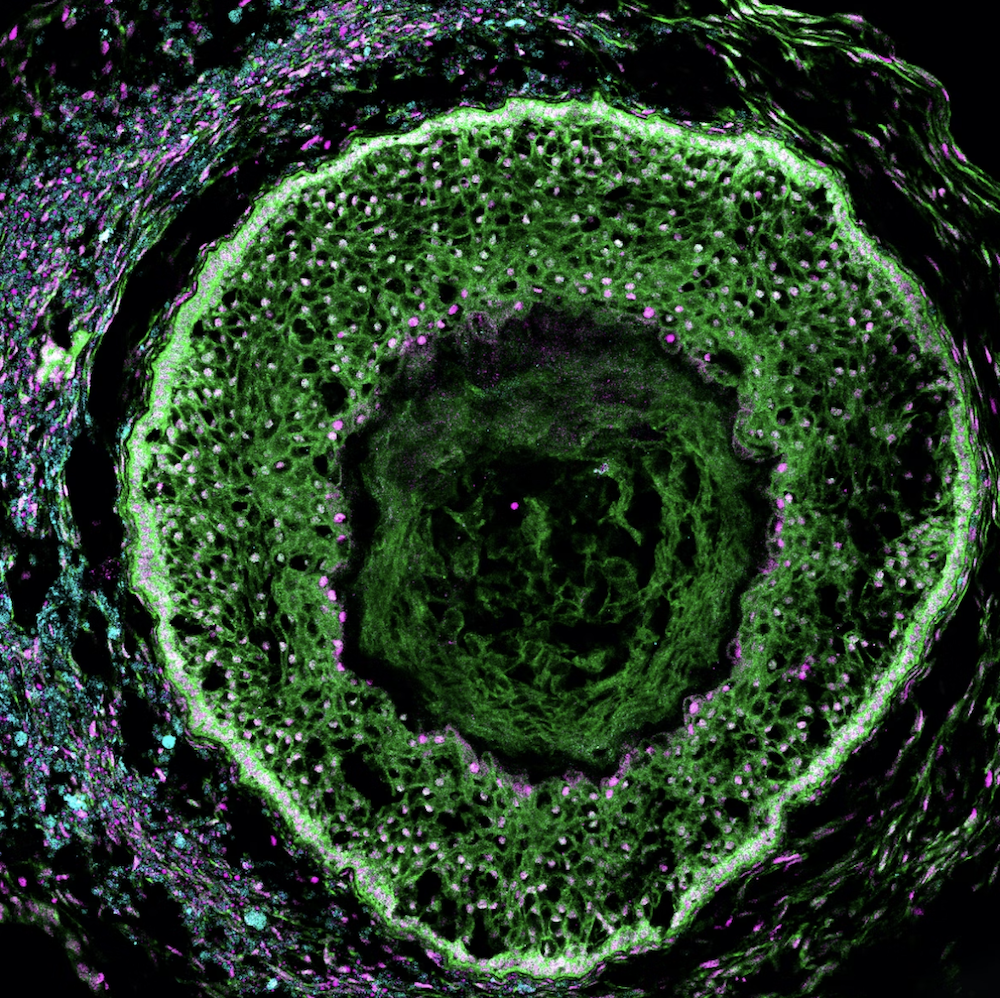
For decades, scientists have been studying the human reproductive system in order to better understand it and develop treatments for various reproductive conditions. Despite this, the ovaries – the organs responsible for producing and releasing eggs in women – have remained surprisingly understudied. Researchers have long sought to create in vitro models of human ovaries, which would allow for more in-depth study of these organs, but most existing models use a combination of human and mouse cells that do not fully replicate the functions of the human ovary and take a long time to grow in the lab.
A recent breakthrough from the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard Medical School, and Duke University in collaboration with biotechnology company Gameto, may change all that. The researchers have successfully created a fully human ovarian organoid that supports egg cell maturation, develops follicles, and secretes sex hormones. This “ovaroid” model enables the study of human ovarian biology without the need to take tissue from patients, and could enable the development of new treatments for conditions like infertility, ovarian cancer, and more.
The ovaroids are described in detail in a new paper published in the journal eLife. Through an agreement with Harvard’s Office of Technology Development, this technology has now been licensed to Gameto, which is using it to develop therapeutics for diseases of the female reproductive system.
“Our new method of fully human ovaroid production is several times faster than existing human/mouse hybrid methods, and replicates many of the critical functions of these organs, marking a significant step forward in our ability to study female reproductive health in the lab. In the future, similar technology could also treat infertility by growing egg cells from people whose own eggs aren’t viable,” said co-first author Merrick Pierson Smela, a graduate student in the lab of George Church, Ph.D. at the Wyss Institute and HMS.
“Our new method of fully human ovaroid production is several times faster than existing human/mouse hybrid methods, and replicates many of the critical functions of these organs, marking a significant step forward in our ability to study female reproductive health in the labIn the future, similar technology could also treat infertility by growing egg cells from people whose own eggs aren’t viable,” said co-author Merrick Pierson Smela, a graduate student in the lab of George Church, Ph.D. at the Wyss Institute and HMS.
“Half of the human population is female, and yet historically women’s health has not received anywhere near the attention or funding that is given to conditions that affect men. I’m very excited to see this important step forward in being able to study human ovaries in the lab, and look forward to the insights that such a model will provide about female reproductive health and disease,” said Wyss Founding Director Don Ingber, M.D., Ph.D. Ingber is also the Judah Folkman Professor of Vascular Biology at HMS and Boston Children’s Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.



