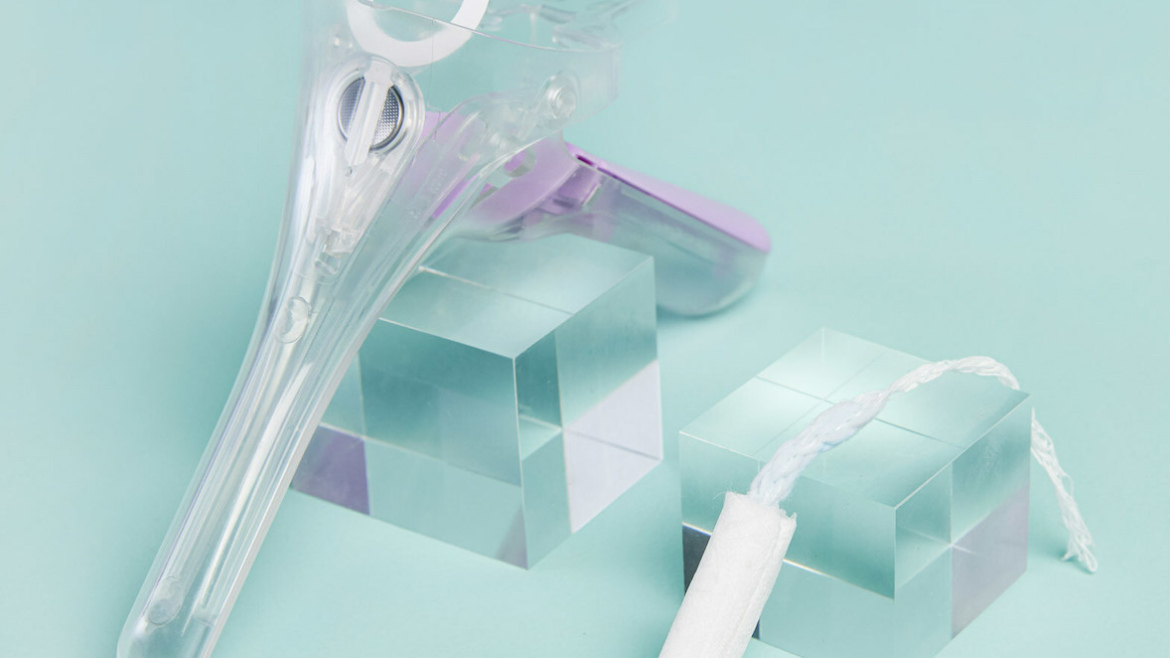
Gynae Health startup Daye and clinical trial company Lindus Health have launched a clinical trial for a Diagnostic Tampon designed to allow women and assigned female at birth (AFAB) individuals to conduct HPV and STI tests in their homes. Supporting Daye’s mission to close the gender health gap, its Diagnostic Tampon has the potential to transform women’s experience when being screened for…